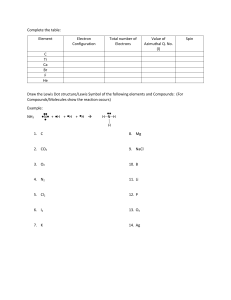
What is the Lewis dot structure of Na+? The Lewis dot structure for Na+ is Na with no dots or electrons and a positive charge written at the top right corner. Explanation: A Lewis dot structure is a diagram that shows the valence electrons of an atom and the way they are arranged in that atom's bonding. In Na+ ion, the sodium ion has lost one electron to become a cation with a positive charge. The electron configuration of Na+ is 1s2 2s2 2p6, which is the same as that of neon. Since there are no valence electrons in Na+, there is no need to draw dots in the Lewis dot structure. The + sign indicates that the ion has a charge of 1+, so it contains one less negative electron than neutral sodium. For reference, the Lewis dot structure of neutral sodium (Na) would show one valence electron represented by a single dot on the right side of the symbol. However, since Na+ has lost an electron, it no longer has any dots in its Lewis dot structure.