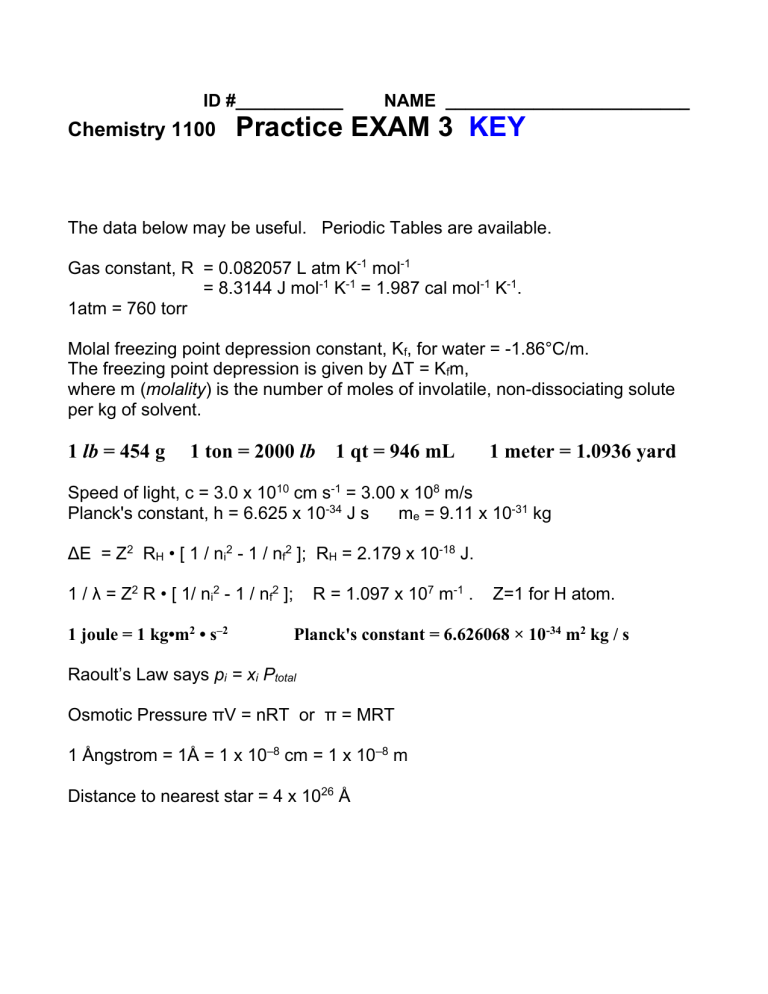
ID #___________ Chemistry 1100 NAME _________________________ Practice EXAM 3 KEY The data below may be useful. Periodic Tables are available. Gas constant, R = 0.082057 L atm K-1 mol-1 = 8.3144 J mol-1 K-1 = 1.987 cal mol-1 K-1. 1atm = 760 torr Molal freezing point depression constant, Kf, for water = -1.86°C/m. The freezing point depression is given by ΔT = Kfm, where m (molality) is the number of moles of involatile, non-dissociating solute per kg of solvent. 1 lb = 454 g 1 ton = 2000 lb 1 qt = 946 mL 1 meter = 1.0936 yard Speed of light, c = 3.0 x 1010 cm s-1 = 3.00 x 108 m/s Planck's constant, h = 6.625 x 10-34 J s me = 9.11 x 10-31 kg ΔE = Z2 RH • [ 1 / ni2 - 1 / nf2 ]; RH = 2.179 x 10-18 J. 1 / λ = Z2 R • [ 1/ ni2 - 1 / nf2 ]; 1 joule = 1 kg•m2 • s–2 R = 1.097 x 107 m-1 . Z=1 for H atom. Planck's constant = 6.626068 × 10-34 m2 kg / s Raoult’s Law says pi = xi Ptotal Osmotic Pressure πV = nRT or π = MRT 1 Ångstrom = 1Å = 1 x 10–8 cm = 1 x 10–8 m Distance to nearest star = 4 x 1026 Å 1. From the mass spectrum shown in the figure, what would you calculate the atomic mass of the element to be? A. 21.2 B. 10.8 C. 10.0 D. 11.0 E. 10.5 majority 11 (about 80%), minority 10 (about 20%) so average about 10.8 2. Consider two bulbs of gas connected by a thin pipe which is opened with the turn of the valve. What will be the final partial pressures of N2, O2 and what will be the total pressure? 6.00 L N2 1.00 atm 4 L O2 1.00 atm A. N2 6atm; O2 4 atm; Ptotal 10 atm B. N2 4atm; O2 6 atm; Ptotal 10 atm C. N2 0.6atm; O2 0.4 atm; Ptotal 6 atm D. N2 0.6atm; O2 0.4 atm; Ptotal 1 atm E. N2 0.06atm; O2 0.04 atm; Ptotal 0.10 atm N2: P1V1=P2V2 1atmx6L=P2x10L P2=0.6atm O2: P1V1=P2V2 1atmx4L=P2x10L P2=0.4atm 3. P outside (right side of the tube) is 760mm Hg. What is the pressure of the gas inside? A. 760 mm Hg B. 510 mm Hg C. 1010 mm Hg D. 250 mm Hg E. - 250 mm Hg 4. Consider a Na+ ion in the NaCl lattice. How many next nearest neighbors (Na+ ) does it have? A. 12 B. 4 C. 6 D. 8 E. 16 5. Consider a Cl– ion in the NaCl lattice. How many nearest neighbors (Na+) does it have? A. 12 B. 6 C. 4 D. 8 E. 16 6. Which of the molecules listed has/have dipole moments? A. CO2 B. C6H6 C. C2H2 D. all of them E. none of them 7. The inert gas Kr forms a liquid –153 °C. This is due to which kind of interaction? A. ion-dipole B. hydrogen-bonding C. ion-ion D. (London) dispersion forces E. covalent bonds 8. A thin layer of snow falls. Next week it’s dry but constantly below freezing. The snow vanishes all by itself. Why? A. It melts as thin layers, contrary to science B. It turns into ice IX which is invisible (see Vonnegut’s book) C. It sublimes – goes straight from solid to water vapor D. It converts into oxygen and hydrogen gas E. This is a mystery about snow that can’t be explained 9. Which of the following is incorrectly classified? A. KH - ionic hydride B. CH4 – molecular hydride C. HI - molecular hydride D. CsH - molecular hydride It’s ionic, Cs+H– E. H2S - molecular hydride 10. Which of the following statements is false? A. The effective nuclear charge experienced by an electron in an outer shell is less than the actual nuclear charge. B. Within a family (vertical group in the periodic table) of representative elements atomic radii increase from top to bottom. C. Electrons in inner shells screen, or shield, electrons in outer shells from the full effect of the nuclear charge. D. The atomic radii of representative elements decrease from left to right across a period (horizontal row in the periodic table). E. Inert gas atoms are larger than the atoms immediately to their left in the periodic table. They’re smaller (decr. left → right) 11. Judging from the electron affinities listed for the atoms below, which is most likely to form an anion? A. Sulfur (S), EA = –200 kJ/mol–1 greatest attraction for an electron B. Phosphorus (P), EA = –72 kJ/mol–1 C. Silicon (Si), EA = –143 kJ/mol–1 D. Aluminum (Al), EA = –43 kJ/mol–1 E. All of them 12. Which one of the following molecules has a dipole moment? A. Cl2 B. H2 C. CO2 D. CH4 E. BrCl no no cancels cancels Br→Cl 13. Which one of the following statements about compounds or polyatomic ions of the A group elements is false? A. All compounds in which the central atom is dsp3 hybridized violate the octet rule. B. Sulfur hexafluoride is an example of a compound with a central atom that has dsp3 hybridization. C. All molecules in which the central element is d2sp3 hybridized have octahedral electronic geometry. D. PF6– is an example of an ion with a central element that has d2sp3 hybridization. E. All ions in which the central atom is dsp3 hybridized have trigonal bipyramidal electronic geometry. 14. Which of the following has an ionic structure? A. CBr4 B. H2S C. NO D. NaBr E. Xe 15. Which of the following forms a covalent solid at room temperature? A. O2 B. NaCl C. A. Na2SO4 D. diamond E. KBr 16. The number of exchangeable hydrogens in H2SO4 and H3PO4 is, respectively….. A. 2, 3 B. 0, 0 C. 1, 2 D. 1, 1 E. 4, 2 17. The number of exchangeable hydrogens in CH3COOH, HCOOH and H2O is, respectively….. A. 2, 4, 2 B. 0, 0, 0 C. 2, 4, 0 D. 1, 1, 2 E. 4, 2, 2 18. Which of the following molecules do(es) not have tetrahedral electronic geometry? A. SF4 B. SO32C. HClO3 D. BF3 E. CH2I2 19. What is the hybridization at phosphorus in PF6– ions? octahedral A. sp B. sp2 C. sp3 D. sp3d E. sp3d2 20. Which of the following molecules has three lone pairs of electrons on the central atom? A. XeF4 B. SF2 C. CO2 D. IF2– E. NF3 ::XeF4 d2sp3 ::SF2 sp3 ::O=C=O:: sp :::IF2– dsp3 :NF3 like NH3 sp3 21. How many grams of sucrose, C12H22O11, are needed to make 4.00 L of 5.00 M solution? [C12H22O11=342 g/mol; a 1.0 M solution contains 1 mole / L] A. 0.0585 g B. 4500 g C. 6840 g D. 342 g E. 7.2 g 4.00L x 5.00mol/L = 20.0 mol. #grams = 20mol x 342 g/mol = 6840 g 22. Balance the following equation. How many H+ are there in the balanced equation? Zn + MnO4– + H+ Zn2+ + Mn2+ + H2O A. 6 B. 8 C. 12 D. 16 E. 24 – + – 2+ MnO4 + 8 H + 5 e Mn + 4 H2O ........... 2 times 2+ – Zn Zn + 2 e .................... 5 times – 5 Zn + 2 MnO4 + 16 H+ 5 Zn2+ + 2 Mn2+ + 8 H2O 23. A. B. C. D. E. Which of the following is right? H2CCH2 sp, triple bond; HCCH sp2, triple bond H2CCH2 sp, triple bond; HCCH sp2, double bond H2CCH2 sp, double bond; HCCH sp2 triple bond H2C=CH2 sp2, double bond; HC≡CH sp, triple bond None of the above is right 24. How many moles of Ca(OH)2 would react with 37.1 mL of 0.138 M HCl? Ca(OH)2 + 2HCl → CaCl2 + 2 H2O A. 0.00511 B. 0.0102 C. 10.2 D. 0.538 E. 0.00256 0.0371 L x 0.138 mol/L = 0.005112 mol HCl = ½ x 0.005112 mol Ca(OH)2 = 0.00256 mol Ca(OH)2 25. At what temperature would a mole of hydrogen occupy 10 L at atmospheric pressure? (R = 0.08206 L atm K–1 mol–1) PV=nRT or T = (PV)/(nR) = (1 atm x 10 L)/(1 mol x 0.08206 L atm K–1 mol–1) = 122 K A. B. C. D. E. 273 K 12.2 K 100 K 12.2°C 122 K 26. At what pressure would a mole of helium occupy 10 L at 0 ˚C ? PV=nRT or P=(nRT)/V=(1 mol x 0.08206 L atmK–1mol–1x273 K)/10L= 2.24 atm A. 1.4 atm B. 0.828 atm C. 4.44 atm D. 22.4 atm E. 2.24 atm 27. The oxidation number of N in the substances Li3N NH3 N2H4 N2 NO HNO2 NO2 HNO3 HN3 , is respectively A. –3 –3 –3 0 2 1 4 5 –1/3 B. –1 –3 –3 0 2 5 0 3 1/3 C. –3 3 –2 0 4 3 4 5 1/3 D. –3 –3 –2 0 2 3 4 5 –1/3 E. –1 –1 –2 2 1 2 6 3 –3 28. What is the hybridization at arsenic in AsF5 molecules? A. sp B. sp2 C. sp3 D. sp3d E. sp3d2 like PF5 trigonal bipyr 29. A sample of carbon monoxide, CO, occupies a volume of 350 mL and exerts a pressure of 1020 torr at 25°C. If the volume expands to 500 mL with no temperature change, what pressure will the gas exert? A. 617 torr B. 827 torr C. 714 torr D. 938 torr E. 1457 torr P1V1=P2V2 350mL x 1020torr = 500mL x P2 P2 = 350x1020/500 torr = 714 torr 30. In a hot air balloon (made of plastic, inflated, open, and with a heater beneath), what’s fixed and what varies? A. P, T and R vary, but not n and V B. V, P and T vary, but n is fixed C. P and n vary, but not T and V D. T and n vary, but not V and P E. V and n vary, but not T and P 31. In an ideal tire full of ideal gas (steel belted, unable to expand no matter how much you blow it up), after air is pumped in extra ideal gas from a tire pump, what’s fixed and what varies? A. P, T and R vary, but not n and V B. V, P and T vary, but n is fixed C. P and n vary, but not T and V D. T and n vary, but not V and P E. V and n vary, but not T and P 32. Which of the following formulas do you expect for an ionic compound formed between barium metal and nitrogen gas? A. Ba2N4 B. Ba3N2 C. Ba2N3 D. BaN E. BaO2 33. A 1.00-L flask contains 2.073 g of O2 and 20.13 g of N2 at 25 °C. What is the total pressure? A. 19.2 atm n=2.073g/32.0g mol-1 + 20.13/28.0g mol-1 =0.7189mol B. 0.856 atm R = 0.082057 L atm K-1 mol-1 C. 1.09 atm P=nRT/V=0.7833molx0.0821L atmK-1mol-1x298K= 19.2atm D. 22.2 atm E. 9.71 atm 34. Based on hydrogen-bonding which of the following should have the highest viscosity? A. CH3OH B. CH3OCH3 C. C2H6 D. C2H2 E. CH2O 35. Arrange in order of increasing ionic radius: Li+ and Be2+ ; Cl– and O2– A. Be2+ < Li+ ; O2– < Cl– B. Be2+ > Li+ ; O2– = Cl– C. Li+ = Be2+ ; O2– = Cl– D. Li+ < Be2+ ; O2– < Cl– E. Atomic radii are always the same 36. Which factor does not influence bond strength? A. Resonance B. Bond multiplicity C. Atomic Radius D. Formal charge FC is a figment of our mathematical imagination E. All of the above influence bond strength 37. What is the molecular geometry of the central atom in CCl4, NH3 and H2O A. CCl4 tetrahedral, NH3 tetrahedral, H2O bent B. CCl4 tetrahedral, NH3 tetrahedral, H2O tetrahedral C. CCl4 square planar, NH3 triangular, H2O bent D. CCl4 square planar, NH3 tripod, H2O bent E. CCl4 tetrahedral, NH3 tripod, H2O bent 38. Which of the following gas molecules should have a dipole moment? A. C2H2 B. H2CCH2 C. H2O D. CO2 E. NH4+ symmetrical H-C≡C-H 39. sym. H2C=CH2 bent H-O-H symm O=C=O symm tetrahedral Which of the following would contain a triple bond? A. C2H4 H2C=CH2 B. H2CCHCH2CH3 H2C=C(H)–C(H)2–CH3 C. H2O H–O–H D. CO C≡O E. N2H4 H2N–NH2 40. Which one of the following salts is insoluble in water? A. AgNO3 B. KI C. NH4Cl D. PbS E. NaBr 41. Which variable is not used to describe the amounts and properties of gases? A. temperature B. pressure C. compressibility D. volume E. number of molecules present 42. All of the following statements, except one, are important postulates of the kinetic-molecular theory of gases. Which one? A. Gases consist of large numbers of particles in rapid random motion. B. The volume of the molecules of a gas is very small compared to the total volume in which the gas is contained. C. The average kinetic energy of the molecules is inversely proportional to the absolute temperature. D. The time during which a collision between two molecules occurs is negligibly short compared to the time between collisions. E. There are no attractive or repulsive forces between the individual molecules. 43. gas? A. B. C. D. E. In the kinetic theory of gases, what gives rise to temperature in a The kinetic energy of the gas molecules Collisions of the molecules with the walls Collisons of the molecules with each other Intermolecular attractions of real gases temperature is proportional to the molecular velocity 44. A 10.0-L flask contains 4.146 g of O2 and 40.26 g of CO at 25 °C. What is the total pressure? A. 19.2 atm n=4.146g/32.0g mol-1 + 40.26g/28.0g mol-1 =1.5674 mol B. 3.84 atm R = 0.082057 L atm K-1 mol-1 C. 1.09 atm P=nRT/V=0.7833molx0.0821L atmK-1mol-1x298K/10.0L= 3.84atm D. 22.2 atm E. 9.71 atm 45. What total volume of gas (at STP) is produced by the electrolysis of 4 moles of H2O? 4 mol H2O → 4 mol H2 + 2 mol O2 = 6 mol gas x (22.4 L/ 1 mol gas) = 134.4 L 2H2O(g) → 2H2(g) + O2(g) A. 67.2 L B. 134.4 L C. 3.0 L D. 112 L E. 5.0 L 46. A sample of hydrogen collected by displacement of water occupied 30.0 mL at 24.0°C on a day when the barometric pressure was 736 torr. What volume would the hydrogen occupy if it were dry and at STP? The vapor pressure of water at 24.0°C is 22.4 torr. A. 32.4 mL P1=736 – 22.4torr P2=760 B. 21.6 mL T1=297K T2=273K C. 36.8 mL V1=30mL V2 = ? D. 25.9 mL P1V1/T1 = P2V2/T2 E. 27.6 mL 47. Which of the following statements is false? A. Solids exhibit vapor pressures, just as liquids do. B. Solids with low vapor pressures sublime easily. C. Dry ice vaporizes at atmospheric pressure and room temperature without passing through the liquid state. D. Deposition occurs when a vapor solidifies without passing through the liquid state. E. Most solids have much lower vapor pressures than liquids do. 48. What is the maximum number of atoms that should bond to a central atom with sp hybridization ? A. None B. One C. Two e.g. C in O=C=O D. Three E. Any number up to infinity 49. At what temperature would a mole of hydrogen occupy 8.21 L at atmospheric pressure? (R = 0.08206 L atm K–1 mol–1, PV = nRT) A. 0.082 K B. 0 K C. 273 K D. 12.2 K E. 100 K PV=nRT or T = (PV)/(nR) = (1 atm x 8.21 L)/(1 mol x 0.08206 L atm K–1 mol–1) = 100 K 50. Which form of the REDOX equation Cr2O72– + Fe2+ + ? → Cr3+ + Fe3+ + ? is balanced correctly? A. Cr2O72– + 14 H+ + 6 e– + Fe2+ → Cr3+ + 7 H2O + Fe3+ B. Cr2O72– + 4 H+ + 6 e– + 3Fe2+ → 7 H2O+ 3Fe3+ C. 2 Cr2O72– + 10 H+ + 3Fe2+ → 2Cr3+ + 5 H2O + Fe3+ D. Cr2O72– + 14H+ + 6 Fe2+ → 2 Cr3+ + 7 H2O + 6 Fe3+ E. None of the above 51. The unit cell of a nitride of vanadium is shown. What is the formula of this salt? A. V2N3 B. VN3 C. V2N D. VN E. V3N2 F. VN2 Called the halite structure, structure of NaCl, etc. Can interchange labels V and N: same structure 52. The unit cell of a metal oxide has M at corners and one M at the center. The other atoms (two inside and four on faces) are oxygen. What is the formula? A. MO B. MO2 C. M2O3 D. M2O E. M2O3 F. M3O2 It is called the rutile structure. It is the structure of the mineral rutile, TiO2 53. The unit cell of a metal oxide is shown. What is the formula? A. MO B. MO2 C. M2O3 D. M2O E. M2O3 F. M3O2 54. Which are endothermic, which exothermic respectively: (i) burning of gasoline; (ii) forming ice from water; (iii) decomposing water to hydrogen and oxygen; (iv) boiling water to steam A. (i) endo (ii) endo (iii) endo (iv) endo B. (i) endo (ii) endo (iii) exo (iv) exo C. (i) endo (ii) endo (iii) exo (iv) endo D. (i) exo (ii) endo (iii) exo (iv) endo E. (i) exo (ii) exo (iii) endo (iv) endo 55. How many moles of NaCl in a 2 mm3 crystal? FW = 58.44, density = 2.17g/cm3. A. 2.17x10-4 B. 7.43x10-5 C. 1.36 D. 1.36x10-7 E. None of these 56. Which of the following diffuses the fastest? A. O2 B. CO C. C8H18 D. C6H6 57. E. SF6 What should be the effect of increasing pressure on the equilibrium 2 NO + O2 ⇌ 2 NO2 A. Shift it to the left B. Increase the yield of oxygen C. Decrease the temperature D. Shift it to the right E. Make it go faster 58. How fast would be the effusion of nitrogen compared to carbon monoxide into space out a small hole in a spacecraft? A. About two times as fast B. About half as fast C. About the same D. About 1.5 times as fast E. These would not effuse 59. Which of the following is one mole? A. 2.24 L of gaseous He at STP B. 12.000 g of 12C C. 35.45g of Cl2 D. 64.0g of O2 E. None of the above 60. What is the length of the bond in CO? Covalent radii: C– 0.77Å; C= 0.67Å C≡ 0.60Å O– 0.74Å; O= 0.60Å O≡ 0.55Å A. 1.51 Å B. 1.27 Å C. 1.15 Å triple bond radii 0.60+0.55 D. 1.37 Å E. 1.32 Å 61. What are the oxidation numbers in H2SO4? A. H +1 S +2 O –2 B. H –1 S +2 O –2 C. H +1 S +6 O –2 D. H +1 S +6 O +2 E. H +1 S +2 O –6 62. Identify the species that have been oxidized or reduced in the reaction 3 Fe2+(aq) + Cr6+(aq) → 3 Fe3+(aq) + Cr3+(aq) A. Fe2+(aq) is oxidized and Cr6+(aq) is reduced B. Fe2+(aq) is oxidized and Cr6+(aq) is oxidized C. Fe2+(aq) is reduced and Cr6+(s) is oxidized D. Fe2+(aq) is reduced and Cr6+(aq) is reduced E. None of the above 63. Unbalanced equation: Cr2O72– + Fe2+ + ? → Cr3+ + Fe3+ + … A. RED: Cr2O72– + 8 H+ + 5 e– → Cr3+ + 4 H2O OX: Fe2+ → Fe3+ + e– B. RED: Cr2O72– + 4 H+ + 5 e– → Fe2+ + 4 H2O OX: Fe2+ → Fe3+ + 2 e– – + – 3+ C. RED: 2 MnO4 + 8 H + 5 e → 2 Cr + 4 H2O OX: Fe2+ → Fe3+ + 2 e– D. RED: Cr2O72– + 14 H+ + 6 e– → 2 Cr3+ + 7 H2O OX: Fe2+ → Fe3+ + e– E. None of the above 64. We use propane for heating. Burning hydrocarbons is an oxidationreduction reaction. Identify the oxidizing agent and the reducing agent. A. C3H8 is the oxidizing agent, O2 the reducing agent B. CO2 is the reducing agent, H2O the oxidizing agent C. C3H8 is the reducing agent, O2 the oxidizing agent D. CO2 is the oxidizing agent, H2O the reducing agent E. None of the above 65. The oxidation number of P in the substances H3PO4 Na3P P2O5 P4 P2O3 K3P7 PF3 H3PO3 Ba3P2 , is respectively A. 5 –3 –5 0 –3 –7/3 3 3 –3 B. –5 –3 –5 0 –3 –7/3 0 –3 3 C. 4 3 –5 0 –3 –7/3 –3 –3 5 D. 5 –3 5 0 –2 7/3 –3 3 –3 E. 5 –1 5 2 –3 3/7 3 –3 –3 66. Give the oxidation numbers of Manganese, Bromine and Nitrogen in MnO2 (Manganese Dioxide), BrF3 (trifluorobromine), and NO3– (nitrate) ion respectively A. +2, –1, –5 B. +2, +3, +5 C. +4, +3, +5 D. +4, –1, +5 E. +4, +3, -5 67. We can solubilize copper with nitric acid, forming blue Cu2+ (aq) and colorless NO gas. What is oxidized / reduced? Cu (s) + HNO3(aq) → Cu2+(aq) + NO(g) A. Cu(s) is reduced, HNO3(aq) is oxidized B. Cu(s) is oxidized, HNO3(aq) is reduced C. Cu2+(aq) is reduced, NO(g) is oxidized D. Cu2+(aq) is oxidized, NO(g) is reduced E. The concept of redox does not apply the copper 68. We dissolve copper in nitric acid, forming blue Cu2+(aq) and NO(g). Which is the oxidizing / reducing agent? Cu (s) + HNO3(aq) → Cu2+(aq) + NO(g) A. Cu(s) oxidizing agent, HNO3(aq) reducing agent B. Cu(s) reducing agent, HNO3(aq) oxidizing agent C. Cu2+(aq) oxidizing agent, NO(g) reducing agent D. Cu2+(aq) reducing agent, NO(g) oxidizing agent E. Cu(s) and HNO3(aq) are always reduced at the same time 69. What are the oxidation states in HNO3 ? A. H = +1, N = +1, O = –2 B. H = +1, N = +1, O = +2 C. H = +1, N = +5, O = –2 D. H = +1, N = +2, O = –2 E. H = +1, N = +6, O = –2 Given RED: Cr2O72– + 14 H+ + 6 e– → 2 Cr3+ + 7 H2O OX: Fe2+ → Fe3+ + e– Which is right …. ? A. Cr2O72– + 14 H+ + 6 e– + Fe2+ → Cr3+ + 7 H2O + Fe3+ B. Cr2O72– + 4 H+ + 6 e– + 3Fe2+ → 7 H2O+ 3Fe3+ C. 2 Cr2O72– + 10 H+ + 3Fe2+ → 2Cr3+ + 5 H2O + Fe3+ D. Cr2O72– + 14H+ + 6 Fe2+ → 2 Cr3+ + 7 H2O + 6 Fe3+ E. None of the above 70. 71. Which is right …. ? A. MnO4 – + 8 H+ + 5 e– → Mn2+ + 4 H2O B. MnO4 – + 4 H+ + 5 e– → Mn2+ + 4 H2O C. 2 MnO4 – + 8 H+ + 5 e– → Mn2+ + 4 H2O D. MnO4 – + 8 H+ + 5 e– → Mn2+ + 4 H2O E. None of the above Cu → 2 Cu2+ + 1 e– Cu → Cu2+ + 2 e– Cu → Cu2+ + 2 e– Cu → Cu2+ + 2 e– 72. The partial equation that describes the oxidation of H2O to O2 is the basis of photosynthesis. Which partial equation is correct? A. O2 + 4 H+ + 4 e– → 2 H2O B. O2 + 4 H+ + 4 e– → O2 +4 H+ + 4 e– C. 2 H2O → O2 + 4 H+ + 4 e– D. 4 H2O + 4 e– → 2 H2O E. 4 H2O + 2 e– → O2 +4 H+ + 4 e– 73. Hydrogen burns in the air to form water. What is the reducing agent, what gets reduced? A. Nitrogen, nitrogen B. Oxygen, hydrogen C. Hydrogen, oxygen D. All of the above E. This is not a redox reaction Given RED: Cr2O72– + 14 H+ + 6 e– → 2 Cr3+ + 7 H2O OX: Fe2+ → Fe3+ + e– Which is right …. ? A. Cr2O72– + 14 H+ + 6 e– + Fe2+ → Cr3+ + 7 H2O + Fe3+ B. Cr2O72– + 4 H+ + 6 e– + 3Fe2+ → 7 H2O+ 3Fe3+ C. 2 Cr2O72– + 10 H+ + 3Fe2+ → 2Cr3+ + 5 H2O + Fe3+ D. Cr2O72– + 14H+ + 6 Fe2+ → 2 Cr3+ + 7 H2O + 6 Fe3+ E. None of the above 74. 75. Which is right …. ? A. MnO4 – + 8 H+ + 5 e– → Mn2+ + 4 H2O Cu → 2 Cu2+ + 1 e– B. MnO4 – + 4 H+ + 5 e– → Mn2+ + 4 H2O Cu → Cu2+ + 2 e– C. 2 MnO4 – + 8 H+ + 5 e– → Mn2+ + 4 H2OCu → Cu2+ + 2 e– D. MnO4 – + 8 H+ + 5 e– → Mn2+ + 4 H2O Cu → Cu2+ + 2 e– E. None of the above 76. Carbon dioxide, CO2 , has a higher boiling point than carbon tetrafluoride, CF4. This can best be explained by …. A. stronger dispersion forces between carbon dioxide molecules than CF4 molecules B. carbon dioxide being polar and while CF4 is not C. stronger carbon-oxygen than carbon-fluorine covalent bonds D. stronger hydrogen bonding between CO2 molecules than in CF4 E. CO2 having greater polarity than CF4 77. The processes of freezing from liquid to solid, and the process of condensation from gas to liquid are, respectively …. A. exothermic, exothermic melting and vaporization would be endothermic B. neither endothermic nor exothermic C. exothermic, endothermic D. endothermic, exothermic E. endothermic, endothermic 78. 79. Which does not affect the amount of heat transferred in solids? A. The kind of solid it is B. The amount of change in temperature C. The specific heat D. The rate at which the temperature changed E. The mass of the solid In which of the following is resonance not possible? A. SO3 B. NO3– C. CO32– D. BF4– E. benzene SO3 , NO3– and CO32– are isoelectronic and each have 1 double bond and 2 single bonds, allowing resonance. BF4– has only single bonds, no resonance 80. A piece of limestone (calcium carbonate MW 100 g/mol) weighing about 200 g is heated till all is converted into calcium oxide and carbon dioxide. How much gas is formed at STP? CaCO3 → CaO + CO2 A. 22.4 L B. 200 L C. 44.8 L D. 100 L E. 57.2 L 1mol CaCO3 → 1 mol CO2 ; 200 g CaCO3 = 2 mol CO2 = 2 mol gas = 2 x 22.4 L at STP 81. Which is not 1 mole? A. 6.02 x 1023 molecules of barium sulfate BaSO4 B. 63.1 g of barium nitrate Ba(NO3)2 1 mol Ba(NO3)2 = MBa + 2MN2 +6MO2 = 261.4 C. Avogradro’s number of cats D. 100.1 g of limestone CaCO3 E. 261.4 g of barium nitrate Ba(NO3)2 1 mol Ba(NO3)2 = MBa + 2MN2 +6MO2 = 261.4 82. Which of the following is the strongest acid? A. HNO3 B. HCOOH C. CaCO3 D. H2O E. NaOH Which of the following is the strongest base? A. HNO3 B. HCOOH C. CaCO3 D. H2O E. NaOH 83. 84. Which of the following is the least polar covalent bond? A. H – H B. H – C C. H – F D. F – Cl E. H – Br 85. Which of the following is the most polar covalent bond? A. H – H B. H – C C. H – F D. F – Cl E. H – Br







