Concentration Units in Chemistry: A Comprehensive Guide
advertisement

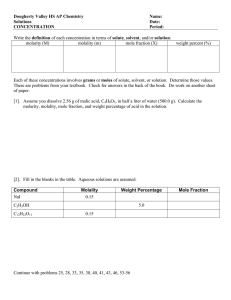
Dr. Masoud DERAKHSHANDEH mderakhshandeh@gelisim.edu.tr Civil Eng. Dep. General Chemistry (CHE102) Common units used for Concentration A sample of blood and Urine analysisi report ni Mole fraction (Xi) = nT Substance a: 2 mol Mass/Volume (gr/l) = Solute mass (g) Solution volume (L) (m/m) (v/v) (m/v) 5 Mass percentage (m/m) Parts per million (ppm)- parts per billion (ppb) ppm and ppb concentrations are essentially mass ratios (solute to solution). Dilute Concentrations Units 7 Example of ppm usage: Carbon monoksit (CO): toxic and lethal dose LCLo value is the lowest concentration of a material in air reported to have caused the death of animals or humans. Multiple Solvent Systems (v/v/v) chloroform/methanol/water 2:2:1.8 (v/v/v) Like: 8 ml Ch + 8 ml met + 7.2 ml Water 9:1 (v/v) ratio of chloroform:methanol Like: 48.2 mL of chloroform, 5.35 mL of methanol 9 0.892 g of potassium chloride (KCl) sample is dissolved in 54.6 g of water. What is the percentage of KCl by mass in solution? Molality (m) Molality is the number of moles of solute dissolved in 1 kg (1000 g) of solvent. Molarity (M) Molarity is the number of moles of solute dissolved in 1 liter (1000 ml) of solution. Why different units are used? • The choice of a concentration unit depends on the purpose of the experiment. • Mole fraction is not used for titrations and gravimetric analysis. • The mole fraction is suitable for calculating the partial pressures of gases. • Molarity is more common because it is usually easier to measure the volume of a solution than to weigh the solvent. • Molality is independent of temperature because it is expressed as the number of moles dissolved and the mass of solvent. • Mass percentage is also independent of temperature. • We do not need to know the molar mass of the solute to calculate the mass percentage. Calculate the molality of a sulfuric acid solution containing 24.4 g of sulfuric acid in 198 g of water. The molar mass of sulfuric acid is 98.09 g. The concentration of K+ in seawater is reported as 10.6 mM. Convert this conc to ppm. Density of seawater is 1.035 kg/L Assumption: we have 1 liter sea water Then: solution mass is: 1 liter × 1.035 kg/L = 1.035 kg =1035 g K+ in seawater : The density of a 2.45 M aqueous methanol solution (CH3OH) is 0.976 g / mL. What is the molality of the solution? The molar mass of methanol is 32.04 g. A solution of methanol, ethanol, water with a proportion of 1:2:10 (v/v/v) are mixed. Calculate the w / w percentage, v / v percentage and w / v percentage ,ppm, molarity, molality, gr / l, and mole fraction of ethanol in this solution. The END Ozone gas, the triple oxygen molecule that acts as a protective stratospheric blanket against ultraviolet rays, is created in nature by lightning. When it strikes, the lightning cracks oxygen molecules in the atmosphere into radicals which reform into ozone. The smell of ozone is very sharp, often described as similar to that of chlorine. This is why you get that “clean” smell sensation after a thunderstorm.