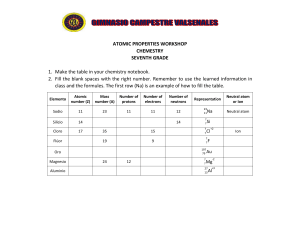
ATOMS, ELEMENTS, MOLECULES COMPOUNDS AND NOMENCLATURE Outline Element and Atom Atomic Number and Atomic Mass Number Ions, Molecules and Compounds Objectives: Be able to: i. Differentiate between the subatomic particles of an atom: • Protons • Neutrons and • Electrons ii. To explain the structure of the nucleus. iii. To identify the atomic number and Atomic Mass Number iv. Identify various atoms and Elements of the Periodic table v. Explain –ve and +ve charges on ionic species of Atoms vi. Name Ions, molecule and compounds An atom is … • Atoms are the basic building blocks of matter. EVERYTHING on Earth is made of atoms… even the air and Your Body! Drugs Water Carbonated Beverages etc.. Atoms are made of three particles: • Protons (+) e• Neutrons (N) e- • Electrons (e-) + N+ N+ +N e- Electron Proton Neutron Protons • Located in the nucleus • Have a positive charge • Have a mass of one Neutrons • Located in the nucleus • Have no charge • Have a mass of one –similar to the proton eN e- Electrons • • • • Have a negative charge Orbit the nucleus of the atom Are very small (have basically NO mass) In a neutral atom, there are the same number of protons and Structure of An Atom Neutron - Electron + Proton + - What force holds all the parts of an atom together? • It is the electromagnetic force of attraction between the positive protons in the nucleus and the negative electrons orbiting around the nucleus that holds the atom together. Lets take a look deep inside ATOM = Proton(s) + Neutron(s) + Electron(s) This is an Electron. - charge eNo Mass + N This is a Neutron. No Charge Mass of ONE This is a Proton. + Charge Mass of ONE Identifies the atom Atomic Number Is the number of PROTONS in the atoms of an element. • It is an IDENTITY of elements on the Periodic Table. • All known elements can be found on the periodic table. • Elements can be identified by their atomic number. • It can be used like a social NRC number for people. Example: An element with 8 protons has an atomic number of 8 and is the element Oxygen from the Periodic Table. Problem Set-1: Class Examples • Identify the element and tell how many protons it has: 1) Atomic number 7 Nitrogen: 7 protons 2) Atomic Number 20 Calcium: 20 protons • Identify the element and give its atomic number. 3) 15 protons 4) 4 protons Phosphorus: Atomic number 15 Beryllium: Atomic Number 4 • Give the atomic number and number of protons. 5) Argon Atomic number 18; 18 protons 6) Sulfur Atomic number 16; 16 protons Atomic Mass Number • Is the total number of Protons and Neutrons of the atom of an element. • Protons and Neutrons are the two largest particles in the atom. • Since they are both located in the nucleus, the mass of the atom is located in the nucleus. Atomic Mass Number = Protons + Neutrons The Periodic Table Atomic Number - Gives number of protons - identifies the element 7 N Element Symbol Gives the name of the element. 14 Atomic Mass Number -The number of protons + neutrons. Commonly, Atomic Mass Numbers are decimal fractions because of Isotopic effects. Isotopes Are atoms of the same element with Same Proton Number But different Neutron Number. 6 6 C C 12 C-12 (98.9%) 6 13 C-13 (1.10%) C 14 C-14 (1ppt) Relative Atomic Mass = m1x1 + m1x1 + m1x1 Relative Atomic Mass = 12x0.989 + 13x0.01 + 14x10-09 Relative Atomic Mass = 12.01 amu Problem Set-2: Class Examples Give the name of the Element, its Atomic Number, Number of Protons, and Atomic Mass Number. Beryllium Atomic number 4 4 protons Atomic mass number 9 Sodium Atomic number 11 11 protons Atomic mass number 23 Oxygen Atomic number 8 8 protons Atomic Mass Number 16 Compounds contain more than one type of atom! Example 1: Organic Compound (a compound with carbon and hydrogen atoms): Methane (natural gas) – CH4 1 atom of carbon and 4 atoms of hydrogen) Example 2: Inorganic compound (a compound without carbon and hydrogen atoms): Water – H2O 2 atoms of hydrogen and 1 atom of oxygen) + K Cl An ion is an atom or group of atoms with a positive or negative charge!! A particle with a neutral charge has the same number of protons and electrons. An ion does not have the same number of electrons and protons. Examples of ions: • H+ - A hydrogen atom that is missing one electron. The atom has one more proton than electron, and must have a positive charge. •CO32- - Carbonate has two more electrons than protons Cl + K Cations and Anions A particle with a positive charge Mn+. Is called a Cation: e.g Fe2+ A particle with a positive charge Xn-. Is called a Anion: e.g H Examples of ions: • H+ - A hydrogen atom that is missing one electron. The atom has one more proton than electron, and must have a positive charge. •CO32- - Carbonate has two more electrons than protons MOLECULES AND COMPOUNDS (NOMENCLATURE) • The inorganic chemical nomenclature was devised by the International Union of Pure and Applied Chemistry (IUPAC). • Precisely identify the chemical composition of the compound. • Based on the Law of Constant Composition Proportions of lements in a compound are ALWAYS the same! Naming Rules For Monoatomic Ion RULES FOR Mn+ : M = Metal, n = oxidation number • If element forms only one cation, we name it by its element name: Na+ = sodium ion Al3+ = aluminum ion • If a transition metal can form more than one cation, add ion’s charge in Roman numerals to the name. Fe2+ is iron(II) ion (ferrous) Fe3+ is iron(III) ion (Ferric). Some Elemental Formulae and Their Ions No.: Element Symbol Ion (s) Name of Ion 1 Sodium Na Na+ Sodium ion 2 Potassium K K+ Potassium ion 3 Calcium Ca Ca2+ Calcium ion 4 Aluminium Al Al3+ 5 Zinc Zn Zn2+ 6 Copper Cu Cu+, Cu2+ Cu(I), Cu(II) 7 Iron Fe Fe2+, Fe3+ Fe(I), Fe(II) 8 Lead Pb Pb2+, Pb4+ Pb(II), Cu(IV) • Name the ion by its element name • For variable cations of the same element, add ion’s charge in Roman numerals to the name. Fe2+ is iron(II) ion (ferrous) Fe3+ is iron(III) ion (Ferric). Ion Names: Classical System Element Formula Lower Charge Name Formula Higher ChargeName Copper Cu+ cuprous Cu2+ Cupric Iron Fe2+ ferrous Fe3+ ferric Lead Pb2+ plumbous Pb4+ plumbic Mercury Hg2+ 2 mercurous Hg2+ mercuric Tin Sn2+ stannous Sn4+ stannic This is for information and for homework only; classical names will not be on quiz or test unless part of a common name that’s required. Some Elemental Formulae and Their Ions No.: Element Symbol Ion (s) Name of Ion 1 Hydrogen H2 H- Hydride ion 2 Nitrogen N2 N3− Nitride ion 3 Oxygen O2 O2- Oxide ion 4 Fluorine F2 F- Fluoride ion 5 Chlorine Cl2 Cl- Chloride ion 6 Bromine Br2 Br - 7 Iodine I2 I- • Take the root of the element name • Add the suffix “-ide” at the end of the name: Common and Systematic Names No.: Common Name Chemical Formula SYTEMATIC NAME 1 Laughing gas N2O Dinitrogen Monoxide 2 Ammonia NH3 Ammonia 3 Table salt NaCl Sodium Chloride 4 Baking soda NaHCO3 Sodium Hydrogen Carbonate 5 Borax Na2B4O7●10H2O Sodium tetraborate decahydrate 6 Hypo Na2S2O3 Sodium Thiosulphate 7 Acetylene C2H2 Ethyne 8 Wood Alcohol CH3OH Methanol 9 Grain alcohol CH3CH2OH Ethanol 10 Vinegar CH3COOH Acetic/ Ethanoic Acid Naming Rules For Monoatomic Ion RULES Xn- IONS: • Take the root of the element name • Add the suffix “-ide” at the end of the name: Cl- = chloride ion Se2- = selenide ion N3- = nitride ion Most often ions are formed when metals combine with nonmetals. (Exception is NH4+ with anion.) THE CHARGE ON AN ION CAN BE PREDICTED FROM ITS POSITION IN THE PERIODIC TABLE elements of Group IA have a +1 charge elements of Group VA have a -3 charge elements of Group IIA have a +2 charge elements of Group VIA have a -2 charge elements of Group VIIA have a -1 charge NAMING IONIC COMPOUNDS RULES i. Always put cation before anion in formula ii. Name of compound is just cation name followed by anion name. EXAMPLES: CATION ANION Ma+ Xb- Ma+b Xb-a Ma+bXb-a MbXa If a = b, then both = 1 by definition for lowest ratio NAMING IONIC COMPOUNDS RULES i. Always put cation before anion in formula ii. Name of compound is just cation name followed by anion name. EXAMPLES: No.: Cation Anion Compound Name of Compound 1 Mg2+ F- MgF2 magnesium fluoride 2 Fe2+ Br- FeBr2 iron(II) bromide 3 Fe3+ Br- FeBr3 iron(III) bromide 4 Ag+ CO32- Ag2CO3 Silver carbonate 5 Ma+ Nb- Ma+bNb-a PROBLEM SET-1: Class Example Write the formula of barium phosphide Step 1. Write down the formulas of the ions. Ba2+ P3Step 2. Combine the smallest numbers of Ba2+ and P3- so that the sum of the charges 3(Ba2+) + 2(P3-) = 0 3(2+) + 2(3-) = 0 The correct formula is Ba3P2 PROBLEM SET-1.2: Class Example 1.2.1 Figure out the charge on each x: a) ZnX, b) NH4X, c) (NH4)2X, d) Al2X3, e) X2(SO3)3 Example 1.2.2: From the about list of compound, identify and write down: a) Compounds where X is cation b) Compounds where X is anion Naming Compounds Containing Polyatomic Ions • A polyatomic ion is an ion that contains two or more elements. E.g NO-3 • Compounds containing polyatomic ions are composed of three or more elements. E.g Na 2CO 3 • They usually consist of one or more cations combined with a negative polyatomic ion. • When naming a compound containing a polyatomic ion, name the cation first and then name the anion. This is the way the formula is written. Na 2CO3 2Na + 23 CO The ions are what is actually present. ANIONS WITH HYDROGEN Some anions have picked up one or two hydrogen ions. Old naming rules put “bi” in front of the anion name. IUPAC uses hydrogen or dihydrogen: CO32- with one H+ added is HCO3Carbonate ion becomes hydrogen carbonate ion. Name these: i. PO43-, ii. HPO42-, iii. H2PO4- One group of ionic compounds that contains all nonmetals is the ammonium salts. Try to write formulas for ammonium sulfate and ammonium phosphate. Four polyatomic ions that do not use the –ate/ ite system. OH - - HS Hydroxide Hydrogen Sulfide CN O - Cyanide 22 Peroxide Binary compounds contain only two different elements. There are four main types, listed in following slides. A. Binary Ionic Compounds Containing a Metal Forming Only One Type of Cation (salts) e.g. Na2O; ZnCl; AlCl3 B. Binary Ionic Compounds Containing a Metal That Can Form Two or More Types of Cations (still salts): FeCl2; FeCl3; CuCl; CuCl2 C. Binary Compounds Containing Two Nonmetals (Binary Covalent) Compounds between nonmetals are molecular, not ionic. N2O; N2O4, H2O D. Acids Derived from Binary Compounds: HCl, HBr, HI A. Binary Ionic Compounds Containing a Metal Forming Only One Type of Cation (salts) e.g. Na2O; ZnCl; AlCl3 Is a metal combined with a non-metal. Name the metal followed by the root name of the nonmetal stem plus the suffix –ide. Here the number of atoms of each element present is not expressed in the name. Practice: CaC2, MgBr2, Al2O3, NaH (calcium carbide, magnesium bromide, aluminum oxide, sodium hydride) B. Binary Ionic Compounds Containing a Metal That Can Form Two or More Types of Cations (still salts) Name the metal Charge in Roman numerals, Name the nonmetal which has stem plus the suffix –ide. Here, the number of atoms of each element present is not expressed in the name. Practice: – – – – FeS: iron (II) sulfide CuCl; Copper (I) chloride SnF2, tin(II) fluoride Mn3(PO4)5: manganese (V) phosphate C. Binary Compounds Containing Two Nonmetals (Binary Covalent) Compounds between nonmetals are molecular, not ionic. – The element that more electropositive (least electronegative) is named first. Rules: Give Greek prefix (to indicate number of atoms of first element) • Don’t use mono prefix for first element. (NO2) Give Greek prefix (to indicate number of atoms of second element) to root of element name, then add -ide Example: N2O3 is dinitrogen trioxide. Exception: hydrogen never has prefix. Some Greek Prefixes S No. Number Greek Prefix A 1 mono B 2 di C 3 Tri D 4 Tetra E 5 penta F 6 hexa G 7 hepta H 8 octa I 9 nona J 10 deca Binary Compounds S No. COMPOUND NAME A N2O3 Dinitrogen trioxide B PCl5 Phosphorous pentachloride (not mono-) C Cl2O7 ------- D CCl4 E CO Carbon tetrachloride Carbon monoxide F CO2 Carbon dioxide G PI3 -------- H H2S Hydrogen sulfide (hydrogen never gets a prefix) I H2O ------ J HCl k ---- phosphorous trichloride l ---- dichlorine oxide D. Acids Derived from Binary Compounds • Certain binary hydrogen compounds, when dissolved in water, form solutions that have acid properties. • The aqueous solutions of these compounds are given acid names. • The acids names are in addition to their –ide names. • Hydrogen is typically the first element of a binary acid formula. D. Acids Derived from Binary Compounds • To name binary acids write the symbol of hydrogen first. • After hydrogen write the symbol of the second element. • Place the prefix hydro- in front of the stem of the nonmetal name. • Place the suffix -ic after the stem of the nonmetal name. Practice: HCl, H2S, HI (hydrochloric acid, hydrosulfuric acid, hydroiodic acid) A. B. C . D . If an ionic compound is a hydrate, it will have •H2O in the formula, like MgCO3•6 H2O Name the ionic compound part using ionic naming rules, Give the Greek prefix for the number of water molecules Add the word hydrate: hexa MgCO3•6H2O Hydrate Magnesium carbonate NOTE: Numbers and corresponding Greek prefixes FORMULA # H2O GREEK PREFIX CLASSICAL NAME SYSTEMATIC NAME 1 CaSO4*2 H2O 2 di gypsum Calcium sulfate dihydrate 2 MgSO4*7 H2O 7 hepta epsom salts Magnesium sulfate heptahydrate 3 Na2CO3*10 H2O 10 deca washing soda Sodium carbonate decahydrate 4 CuSO4*5 H2O 5 penta bluestone Copper(II) sulfate pentahydrate At this level, no naming rules at all Just learn formulas and names. CH4 methane C3H8 propane C8H18 octane CH3OH methanol CH3CH2OH ethanol CH3CHOHCH3 Propanol COOH Formic acid CH3COOH acetic acid C6H12O6 glucose CH3NH2 aminomethane C12H22O11 sucrose C2H2 ethyne