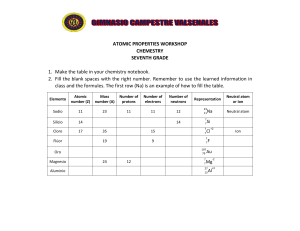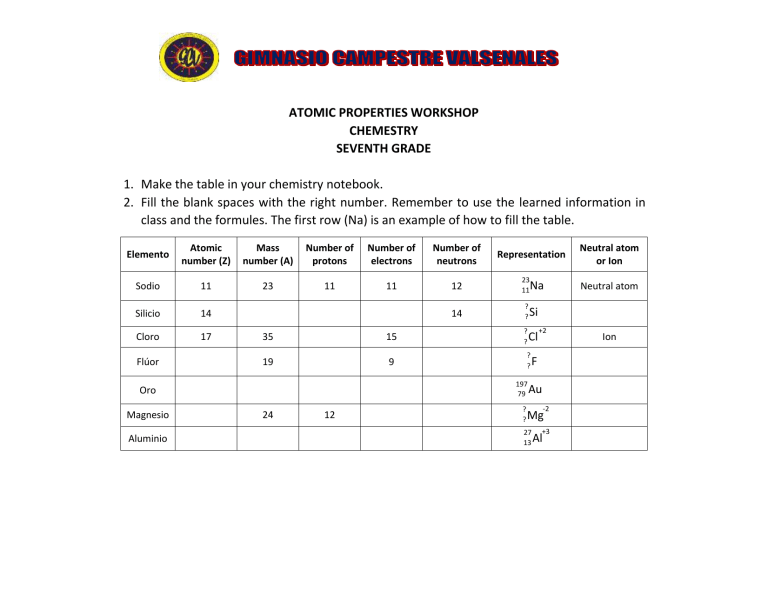
ATOMIC PROPERTIES WORKSHOP CHEMESTRY SEVENTH GRADE 1. Make the table in your chemistry notebook. 2. Fill the blank spaces with the right number. Remember to use the learned information in class and the formules. The first row (Na) is an example of how to fill the table. Elemento Atomic number (Z) Mass number (A) Number of protons Number of electrons Number of neutrons Sodio 11 23 11 11 12 23 11 Silicio 14 14 ? ? Cloro 17 Flúor 35 15 19 9 Aluminio ? ? 24 12 Na ? ? Neutral atom or Ion Neutral atom Si Cl ? ? 197 79 Oro Magnesio Representation +2 F Au -2 Mg 27 13 +3 Al Ion