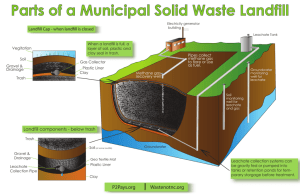
Leachate Quality Analysis and Passive Treatment Options Pema Choden Master of Philosophy (Environmental Science) A Thesis submitted for the fulfilment of M Phil. Degree, The University of Newcastle, N.S.W. July, 2011 “I hereby certify that the work embodied in this thesis is the result of original research and has not been submitted for a higher degree to any other University or Institution.” Signed …………………………… Acknowledgements First of all, I would like to gratefully and sincerely thank Associate Proffessor Phillip Geary for his continuous guidance, support and patience. He has contributed significantly in gaining new experiences and immense knowledge in my life. I would also like to thank Mr Joe Whitehead for his assistance as a co-supervisor and for providing valuable discussions and accessibility. I have deep appreciations to both of them for being very kind, co-operative and understanding throughout the study period. Secondly, I would like to thank Dr Kim Edmunds for her immense support in editing. Dr Kim worked hard and spent much time reviewing the draft chapters of the thesis. She also gave me necessary inspiration to have faith in myself while writing the thesis. I am grateful to Dr Steven Lucas, Mr Richard Bale, Mr Christopher Dever and Mr Matthew Davies for all the laboratory assistance whenever needed. To all my friends in Geology 109, they have provided me some much needed company, entertainment and humour in what could have otherwise been a stressful study environment. I always felt happy to have them around and talk to them whenever depressed. Finally, and especially, for my parents (Dad, Mom and Sister, Pema Wangmo), I thank you for the unwavering support, prayers and strong faith in me and for being tolerant while being away from home, over a long period. Without them, this would not have been possible. Table of Contents Acknowledgements .................................................................................................................... i Table of Contents ..................................................................................................................... ii List of Tables ........................................................................................................................... iv List of Figures ........................................................................................................................... v List of Equations ..................................................................................................................... vi Abbreviations, Acronym and Symbols ................................................................................. vii Abstract .................................................................................................................................... ix Chapter 1 Introduction ............................................................................................................ 1 1.1 Waste Management in Bhutan.................................................................................... 4 1.2 Research Aims .............................................................................................................. 5 1.3 Thesis Approach and Structure .................................................................................. 6 Chapter 2 Background ............................................................................................................ 8 2.1 Solid Waste Management Techniques ....................................................................... 8 2.1.1 2.1.2 2.1.3 2.2 Composting ..................................................................................................................................... 8 Incineration.................................................................................................................................... 12 Landfills ........................................................................................................................................ 13 Review of Leachate Treatment Options ................................................................... 19 2.2.1 2.2.2 2.2.3 2.2.4 Aerobic biological treatment (Aeration) ........................................................................................ 19 Flocculation ................................................................................................................................... 22 Membrane separation .................................................................................................................... 24 Activated Carbon Adsorption (ACA) ............................................................................................ 24 Chapter 3 Solid Waste Management .................................................................................... 26 3.1 Waste Management in Bhutan.................................................................................. 26 3.2 Summerhill Waste Management Centre (SWMC), Newcastle .............................. 28 3.3 Leachate Characterization and Statistical Evaluation ........................................... 30 3.3.1 3.4 Temporal variation in leachate quality .......................................................................................... 32 Outcomes of Leachate Characterization and Evaluation ...................................... 34 Chapter 4 Materials and Methods ........................................................................................ 41 4.1 Aerobic Biological Treatment ................................................................................... 41 4.1.1 4.1.2 4.1.3 4.1.4 4.2 Site selection ................................................................................................................................. 41 Physical and chemical parameters of the leachate (sample) .......................................................... 41 Characterisation of leachate sample .............................................................................................. 44 Set-up of the aeration experiment .................................................................................................. 45 Column Experiments ................................................................................................. 45 4.2.1 4.2.2 4.2.3 Setup of the column experiments .................................................................................................. 45 Characterisation of leachate sample .............................................................................................. 47 Determination of physical and chemical properties of the filter materials .................................... 47 4.3 Materials for Leachate Characterisation and Evaluation ...................................... 51 Chapter 5 Results ................................................................................................................... 52 5.1 Aerobic Biological Treatment ................................................................................... 52 5.1.1 5.1.2 5.1.3 5.1.4 5.1.5 5.2 Change in the nitrogen compounds ............................................................................................... 54 Turbidity ....................................................................................................................................... 56 Electrical conductivity (EC) .......................................................................................................... 58 pH and total alkalinity .................................................................................................................. 59 Phosphorus .................................................................................................................................... 60 Column Experiments ................................................................................................. 61 5.2.1 5.2.2 5.2.3 5.2.4 5.2.5 Solube reactive phosphorus removal ............................................................................................ 66 Turbidity and Colour ..................................................................................................................... 69 Electrical conductivity ................................................................................................................... 71 Total alkalinity and pH .................................................................................................................. 72 Ammonia-nitrogen, Nitrite-nitrogen and Nitrate-nitrogen ............................................................ 74 Chapter 6 Discussion ............................................................................................................. 76 6.1 Aerobic Biological Treatment ................................................................................... 76 6.1.1 6.1.2 6.1.3 6.1.4 6.1.5 6.1.6 6.1.7 6.1.8 6.2 Ammonia-nitrogen removal .......................................................................................................... 77 Turbidity and electrical conductivity ............................................................................................. 79 Colour and odour ........................................................................................................................... 80 Phosphorus .................................................................................................................................... 80 Temperature .................................................................................................................................. 81 Retention time ............................................................................................................................... 81 Application .................................................................................................................................... 83 Drawbacks of the experiment ........................................................................................................ 84 Column Experiments ................................................................................................. 85 6.2.1 6.2.2 6.2.3 6.2.4 6.2.5 6.2.6 P-removal ...................................................................................................................................... 85 The effect of chemical composition .............................................................................................. 86 Contact time & hydraulic conductivity ......................................................................................... 87 Longevity ...................................................................................................................................... 88 Particle size distribution, porosity percentage (%) and bulk density ............................................. 88 Drawbacks of the experiment ........................................................................................................ 89 Chapter 7 Conclusions and Recommendations ................................................................... 91 7.1 Laboratory Aeration Experiments ........................................................................... 91 7.2 Laboratory Column Experiments ............................................................................ 91 7.3 Leachate Characterisation ........................................................................................ 92 7.4 Solid Waste Management Options for Bhutan........................................................ 93 7.5 Recommendations and Further Research ............................................................... 94 References ............................................................................................................................... 95 Appendix A ................................................................................................................................ I Appendix B ............................................................................................................................. VI List of Tables Table 2.1 Classification of Landfill Leachate According to the Composition Changes. ...... 16 Table 2.2 Landfill Gas Composition. .................................................................................... 17 Table 3.1 Composition of Landfill Leachate (1995-2009) from SWMC, Newcastle, Australia. ............................................................................................................... 31 Table 3.2 Correlations Between Rainfall in mm and Leachate Volume in KL: i) monthly (2001-2009) and ii) annual (1995 -2009). ............................................................. 39 Table 3.3 Spatial and Altitudinal Variation in Annual Rainfall by Region and Percentage of Total Area in Different Altitudinal Zones in Bhutan. ........................................... 40 Table 4.1 Initial Physio-Chemical Characteristics of the Raw Leachate Sample (11th February 2010). ..................................................................................................... 45 Table 4.2 Initial Physio-Chemical Characteristics of the Raw Leachate Sample and DeIonised Water Used in Column Experiments (18th May 2010). ....................... 48 Table 4.3 Typical Chemical Composition of BFS (amorphous-0.25-4 mm). ....................... 50 Table 5.1 The Change in Characteristics of Landfill Leachate in the two Tanks operated at 21ºC for 20 days. Tank A was the control while Tank B was aerated. ................. 53 Table 5.2 Physical Properties of Three Different Filter Materials: GAC, BFS and sand. .... 63 Table 5.3 Operating Parameters for the Column Experiments. ............................................ 63 Table 5.4 Mean Results Obtained from Column Experiments using three Different Filter Materials. ............................................................................................................... 67 List of Figures Figure 2.1 Typical temperature curve observed during different compost phases.. ............... 10 Figure 2.2 A sketch of a MSW landfill construction.. ............................................................ 14 Figure 2.3 A cross section of a leachate collection system.... ................................................ 17 Figure 2.4 A landfill gas collection and recovery system. ..................................................... 18 Figure 2.5 An aerated lagoon plant......................................................................................... 22 Figure 3.1 Average percentage composition of MSW in Bhutan.. ......................................... 27 Figure 3.2 Frequency distribution curve of ammonia between 1995 and 2009. .................... 35 Figure 3.3 An illustration of six month moving average – leachate percolation versus rainfall (January 2001- December 2009). .......................................................................... 36 Figure 3.4 An illustration of the annual leachate percolation versus annual rainfall received at the landfill site (1995-2009). ............................................................................. 37 Figure 3.5 A linear relationship between rainfall (mm) and leachate volume (KL): a) monthly (R2= 0.238) and b) annual (R2= 0.367). ................................................................ 38 Figure 3.6 A plot of variation in rainfall with changes in season and altitude in Bhutan. ..... 40 Figure 4.1 SWMC landfill and methane collection facility. ................................................... 42 Figure 4.2 Set up of the aeration experiment using glass-sided tanks and aerators showing Tank A (left) and Tank B (right). .......................................................................... 46 Figure 4.3 Setup of the column experiment showing: a) different columns and b) constant head apparatus. ...................................................................................................... 47 Figure 5.1 The changing pattern of ammonia concentration in Tank A and Tank B over a period of 20 days. .................................................................................................. 54 Figure 5.2 The changing pattern of nitrite concentration in Tank A and Tank B over a period of 20 days. ............................................................................................................. 55 Figure 5.3 The changing pattern of nitrate concentration in Tank A and Tank B over a period of 20 days. ............................................................................................................. 56 Figure 5.4 The changing pattern of turbidity in Tank A and Tank B over a period of 20 days. 57 Figure 5.5 The changing pattern of EC in Tank A and Tank B over a period of 20 days. ..... 58 Figure 5.6 The difference in colour of the leachate samples from Tank A (left) and Tank B (right). .................................................................................................................... 59 Figure 5.7 The changing pattern of pH in Tank A and Tank B over a period of 20 days. ..... 59 Figure 5.8 The changing pattern of alkalinity in Tank A and Tank B over a period of 20 days. 60 Figure 5.9 The changing pattern of phosphorus in Tank A and Tank B over a period of 20 days........................................................................................................................ 61 Figure 5.10 Plot of % of media finer by weight as a function of particle size (mm). .............. 64 Figure 5.11 The changing level of phosphorus in the leachate samples following different runs throughout the experiment. ................................................................................... 66 Figure 5.12 P-sorption isotherms for three different filter materials developed using five different P-solutions in the batch scale experiment (Lanfax Laboratories). ......... 69 Figure 5.13 The changing level of turbidity in the leachate samples following different runs throughout the experiment. ................................................................................... 70 Figure 5.14 The difference in the colour of the leachate samples for three different runs along with their respective controls. a) GAC, b) BFS and c) sand. ................................ 71 Figure 5.15 The changing level of EC in the leachate samples following different runs throughout the experiment. ................................................................................... 72 Figure 5.16 The changing level of alkalinity in the leachate samples following different runs throughout the experiment. ................................................................................... 73 Figure 5.17 The change in pH values of the leachate samples following different runs throughout the experiment. ................................................................................... 74 Figure 5.18 The changing level of ammonia in the leachate samples following different runs throughout the experiment. ................................................................................... 74 Figure 5.19The changing level of nitrate in the leachate samples following different runs throughout the experiment. ................................................................................... 75 List of Equations Equation 4.1 Calculation of filter material % retained on sieve.. ............................................ 49 Equation 4.2 Calculation of % finer by weight. ...................................................................... 49 Equation 4.3 Calculation of hydraulic conductivity of the filter material.. ............................ 49 Abbreviations, Acronym and Symbols Abbreviation Term SWM Solid Waste Management MSW Municipal Solid Waste SWMC Summerhill Waste Management Centre AWQGFMW Australian Water Quality Guidelines for Fresh and Marine Waters. BOM Bureau of Meteorology RGoB Royal Government of Bhutan BFS Blast Furnace Slag GAC Granular Activated Carbon PAC Powdered Activated Carbon DO Dissolved Oxygen BOD Biochemical Oxygen Demand COD Chemical Oxygen Demand TOC Total Organic Carbon XOCs Xenobiotic Organic Compounds OM Organic Matter NCM Non-compostable Materials TKN Total Kjeldahl Nitrogen SRP Soluble Reactive Phosphorus Ksat Saturated hydraulic conductivity (permeability) D10 Effective size of filter material finer by 10% LEL Lower Explosive Limit kL Kilolitre EC Electrical Conductivity pH measure of H+ activity UMR Under Measuring Range NA Not Applicable P Phosphorus CH4 Methane CO2 Carbon dioxide NH3 Ammonia CaCO3 Calcium Carbonate Abstract More than 90% of municipal solid waste (MSW) in developing countries is disposed of in landfills. In Bhutan, about 90% of solid waste is disposed of in landfills. One of the significant problems associated with landfills is the generation of leachate. Landfill leachates are highly contaminated waste waters containing high concentrations of organic matter (OM) measured as biochemical oxygen demand (BOD) and chemical oxygen demand (COD), ammonia, halogenated hydrocarbons and trace elements. The direct release of leachate into the environment may pose potential risks and hazards to public health and ecosystems. As a result, cost effective and environmentally acceptable treatments of leachate are sought. This research aims to examine leachate characteristics and two low cost passive treatment options. The characteristics of a typical leachate generated at a modern sanitary landfill were investigated by analysis of long-term monitoring data collected at Summerhill Waste Management Centre (SWMC), Newcastle, NSW. Leachate production from the SWMC landfill was clearly related to rainfall events at the landfill site. Rainfall has a direct impact on the volume of leachate produced and consequently on its chemical characteristics. Thus, leachate treatment systems must have provisions for the variation in concentrations. The primary goal of this study was to investigate the suitability of two low cost passive leachate treatment options, which are viable and suitable for adoption in Bhutan. Two laboratory bench scale experiments were undertaken. The first experiment involved surface aeration of raw leachate over a period of 20 days, while the second investigated the treatment performance of three low cost filter media (Granular Activated Carbon (GAC), Blast Furnace Slag (BFS) and sand) by examining their sorption efficiencies in a series of column experiments. Leachate samples were collected from the landfill at SWMC. The results of the research showed that a medium strength landfill leachate can be treated by both methods to reduce the concentrations of certain parameters. Aerobic treatment enhanced the leachate quality mainly through removal of ammonia and OM (>95%). It resulted in significant pollutant reductions as opposed to no aeration, which resulted in anoxic conditions. Column experiments provided leachate treatment essentially by lowering soluble reactive phosphorus (SRP) concentration. BFS and GAC have performed comparatively better with P-removal efficiencies of 92% and 67%, respectively, than sand (40%) in the laboratory work undertaken. Finally, the research results also suggested landfills in Bhutan do not have appropriate leachate and gas collection facilities. Due to a lack of proper waste segregation, the leachates produced in landfills could be chemically complex. Composting is suggested as a sustainable alternative for SWM in Bhutan to reduce the 50-60% of organic waste disposed of in the landfills if leachate collection and treatment cannot be afforded. Chapter 1 Introduction A large number of reports on Solid Waste Management (SWM) from many countries indicate that sanitary landfill is still the most affordable disposal option for solid waste, especially in the developing countries. About 90% of solid wastes generated in the world are disposed in landfills, for reasons of convenience and affordability. Despite the availability of many alternatives, land filling (variously called a garbage dump or rubbish dump) of Municipal Solid Waste (MSW) is still the main method of disposing solid waste in most countries across Asia, Africa, and Europe. In Asia alone, about ten countries dispose more than 70% of their MSW in landfills ( Autret et al., 2007; Henry et al., 2006; Lopez et al., 2010). Landfills mostly vary in structure, design and management based on the social, technical and economic development status of that country. While some landfills remain simple and traditional, and pose an adverse impact on the environment, others developed into modern sanitary landfills ensure minimal environmental impact. However, a common and major drawback associated with all the landfills is the generation (formation) of heavily polluted leachates (Christensen et al., 2001; Mehmood et al., 2009; Renou et al., 2008). The formation of leachate begins in the landfill where the waste gets mixed up with water from the air and moisture from waste and ground water. Immediately, waste starts to undergo physical, biological and chemical decomposition under aerobic and anaerobic - conditions, producing a large volume of liquid called leachate. Thus, leachate consists of two components: one, a liquid generated by the breakdown of waste in the landfill; and two, the infiltration of precipitation that has percolated through the landfill (Duggan, 2005). Leachates are generally characterised by dissolved contaminants, volatile organic acids, trace elements, and high concentrations of organic matter (high biochemical oxygen demand (BOD) and chemical oxygen demand (COD)) and ammonia (Tyrrel et al., 2002). However, the compositions can vary depending on the nature of waste, the active 1 microbial flora and the characteristics of the soil, the rainfall pattern and the age of the landfill (Cecen et al., 2003; Kargi & Pamukoglu, 2004; Mehmood et al., 2009). When a leachate containing high concentrations of dissolved contaminants is directly released into the environment, it may contaminate soil and water bodies, and may pose potential risks and hazards to the environment and to public health. For instance, numerous cases of contamination of streams, creeks and ground water have been associated with leachate contamination from landfill sites (Christensen et al., 2001; Duggan, 2005; Lim et al., 2009; Pivato & Gaspari, 2006; Robinson et al., 2005; Yang et al., 2008). The high organic compounds, heavy metals and ammonia present in the leachate are identified as potential sources of ground and surface water contamination. Furthermore, if leachates are discharged directly to municipal wastewater treatment plants, they may affect chlorine disinfection efficiency, cause corrosion of the pump station, and sludge build up and settling problems. As a result, the treatment of leachate is necessary before discharging to the environment. Amongst many other factors, the successful treatment or appropriate remedial action for leachate will depend on how its characteristics are understood. In fact, leachate characterization is pivotal in evaluating the environmental risks associated with it. In this regard, a part of this research involved the study of leachate formation and its characteristics at a modern sanitary landfill in Australia, where best management practices are used. Located on an old mine site, the sanitary landfill (Summerhill Waste Management Centre) is located near Newcastle, NSW. It receives four different types of wastes: inert, solid, industrial and hazardous waste. Accordingly, the landfill is divided into different cells, where different waste can be processed and deposited in these respective cells. The main feature of this landfill comprises a series of discrete sections called “cells” lined with an impermeable leachate barrier, which captures the leachate for appropriate disposal. A landfill compactor is used to compact the waste and it is covered daily with approximately 150 mm of cover material (soil). Other important features include provisions for recycling, sorting and gas and leachate collection facilities. 2 In addition, recent studies indicate that apart from the type of waste that goes into the landfill, the volume of water that enters the landfill has an important bearing on the characteristics of the leachate (Tatsi & Zoubolis, 2002). A low strength and dilute leachate is produced when the water percolation is high, whereas, lesser volumes of water percolating through the landfill resulted in high strength leachates with concentrated dissolved contaminants. This phenomenon indicates that water is the most important element in leachate formation. Although, surface drainage and irrigation water are considered to be some of the sources of water, precipitation (rainfall) is the most dominant factor in the volume of leachate produced at a landfill site (Tatsi & Zoubolis, 2002). To enable further understanding of this concept, an overview of the long term leachate monitoring data from a modern sanitary landfill (SWMC) is presented in this study. The study took into account both temporal and long term changes of the leachate characteristics. Further discussions were made regarding the change in leachate characteristics, the volume percolated and the climatic factors (rainfall) at the site to assess the likely correlations existing between them. Generally, there are various treatment methods or remedial actions that have been developed in recent decades aimed at minimising the concentration of the contaminants. The remedial methods in landfills rely on a range of physical, chemical, physiochemical, or biological processes depending on the method used. Physical processes in landfills include dilution (Christensen et al., 2001) whereas physiochemical and chemical processes involve processes such as sorption (Foo & Hameed, 2009), filtration, ion exchange, precipitation and flocculation (Amokrane et al., 1997; Nehrenheim et al., 2008). Biological remedial actions mostly deal with aeration and microbiological (degradation) processes. While many of these methods are now known to be highly successful and efficient, most of them involve huge capital investment and require extensive use of resources and technology, which makes them less than or not feasible in many places, particularly in developing countries where financial constraints and resource limitations are already a problem. 3 1.1 Waste Management in Bhutan Bhutan is a small land-locked country situated between India and China and bordered by Nepal and Bangladesh in the west. It has a total land area of 38,394 km2 and a population of approximately 873,700 persons. Virtually all of Bhutan is mountainous with an elevation of 100 m above sea level in the south to over 7500 m in the north. Three major landform features are evident in Bhutan: the southern foothills; the inner Himalayas; and the high Himalayas featuring snow capped mountains (Uddin et al., 2007). Water and forests are Bhutan’s renewable energy resources and the country depends highly on subsistence farming and hydroelectricity. The major land uses in Bhutan are agriculture (>80% of land use) and forestry. One of the most important threats to Bhutan’s environment is the problem of ever increasing solid waste (Penjor, 2008; Phuntsho et al., 2008; Uddin et al., 2007). While the magnitude of the problem is relatively small and manageable in rural areas, it is growing significantly in urban areas in recent time subsequently posing threats to the environment. The increase in waste generation is primarily attributed to factors such as rapid rates of urbanisation, rural urban migration (6.7%), changing consumption pattern and high population growth rate (11%). As per the national survey of solid waste conducted between 2007 and 2008, mean waste generation has been estimated at approximately 0.5 kg/person/day (Phuntsho et al., 2008). By comparison, the per capita waste generation is still smaller than other countries - North America (2.0 kg/day/person), China (1.59 kg/day/person), Australia (just over 1.0 kg/day/person) and Europe (0.6 kg/day/person) (EPA, 2008; cited in Farrell & Jones, 2009). For instance, Thimphu is the largest city and the capital of Bhutan. The population in Thimphu rapidly increased from 25,000 in 1992 to 80,000 in 2005. Consequently daily waste generation increased by four fold from 17.5 tonnes in 1992 to 64.5 tonnes in 2007 (Penjor, 2008). Similar situations were observed in the three other urban centres Phuentsholing, Paro and Samtse. In the absence of incineration and combustion, land filling of solid waste is the most common method of solid waste disposal in Bhutan. The majority of the country 4 disposes of solid waste in the landfill-like structures, which includes open dumps in narrow valleys, streams and rivers. Due to the lack of vacant land, finance, technology and labour, the landfills do not have proper design, facilities and leachate treatment systems. Hence, leachate contamination at landfill sites is a significant environmental issue. For example, the biggest landfill in the capital (Memelakha Waste Disposal Site) is already overflowing (Allison, 2008; Penjor, 2008).Without proper leachate and gas collection facilities, the issues of free flow of leachate in the environment and release of toxic gases (e.g. methane) in the atmosphere are of concern. In the past few years, several forest fires have been reported to have occurred near the landfill site and methane combustion has been suggested as one of the possible causes of each incident. Given the situation in Bhutan, which is similar in many developing countries, this research has been undertaken to examine a number of simple, effective and affordable treatment measures to reduce leachate contaminant concentrations, which are an important environmental issue. 1.2 Research Aims Although aeration is used for treating wastewater, so far very little is known about its application in the field of leachate treatment (Mehmood et al., 2009; Robinson & Grantham, 1988). Few studies have shown the efficacy of the aeration process in treating leachate, and those that did have used complex processes that are less efficient in terms of cost, resource and application for large scale treatment (Berge et al., 2006; Mehmood et al., 2009; Shao et al., 2008). Similarly, a group of researchers indicated the potential ability of filter materials, both natural and manmade products, for treatment of water, wastewaters and leachates through bench scale column studies (Johansson, 1999; Mohan & Gandhimathi, 2009; Nehrenheim et al., 2008). According to their studies, limestone, blast furnace slag, sand, coal, peat and pine bark are some of the potential media capable of adsorbing and reducing contaminant concentrations in wastewater. However, not much is known with regard to their application in leachate treatment. Previous studies suggest further research on the use of these materials in leachate treatment would not only assist in 5 treating leachate, but also enable the use of industrial by-products like slag and coal (Johansson, 1999; Mohan & Gandhimathi, 2009; Nehrenheim et al., 2008). The main part of this research has evaluated the suitability of two low cost passive treatment methods for treatment of landfill leachates. The first experiment involved aerobic biological treatment (aeration) of leachate in two aeration tanks (25 L) over a period of 20 days, while the second, a column experiment, investigated the suitability of three low cost filter media in treating leachate, by examining their sorption efficiencies and therefore, their capacity to reduce contaminant concentrations. The results from this research will enable recommendations to be made regarding SWM in Bhutan and provide low cost and simple solutions for leachate treatment. To achieve this, the following work was undertaken as part of this research: To review different SWM techniques prevalent in other countries and suggest an improved system of disposing solid waste for Bhutan; and To be able to determine two low cost passive leachate treatment options, which are suitable, environmentally friendly and economically viable for Bhutan. 1.3 Thesis Approach and Structure This thesis is divided into seven different chapters. Chapter One is the introduction to the thesis and covers the main topics to be discussed, the aims of the research and the structure of the thesis. Chapter Two is the background of the thesis and is divided into two parts. The first half of the chapter reviews the prevailing SWM techniques in different countries, their advantages and disadvantages. The second part introduces leachate, its impact on the environment and low cost passive treatment options that have been so far adopted in treating leachate. Chapter Three compares in brief the waste management system in Bhutan to that of Summerhill Waste Management Centre (SWMC) in Newcastle. In addition, this chapter presents an overview of the main physio-chemical characteristics of leachate formed at the SWMC sanitary landfill during the past 14 years. The outcome of the statistical evaluation of long term leachate monitoring data set is discussed in relation to leachate 6 formation at the landfill. Further correlations between the volume of leachate formed and the amount of rainfall received at the site is applied in estimating leachate formation in Bhutan. Chapter Four essentially describes the methodology and materials used in the two bench scale experiments: a) the effect of aerobic biological treatment on leachate quality improvement; and b) column experiments using three different filter media to determine the suitability of low cost filter materials in leachate treatment methods. One approach of the study includes an analysis of long term leachate monitoring data obtained from the SMWC landfill along with climate data obtained from Bhutan and Australia. Chapter Five comprises two main sections dealing with results obtained from the respective bench scale laboratory experiments in relation to their impact on leachate quality improvement. Results of both experiments are based on nine important parameters of leachate. Both experiments evaluated the possibility of treating leachate quality in a simple, affordable and effective way. Chapter Six is divided into two sections. The changes in the quality of the leachate from both bench scale leachate treatment experiments are discussed in detail. The application and limitations of this treatment study including concerns with respect to their application as an onsite leachate treatment plant in Bhutan is outlined. Lastly, Chapter Seven summarizes and concludes this thesis by highlighting the outcomes of the study in relation to its application in treating leachate in Bhutan. Some future research directions are also discussed towards the end of the chapter. 7 Chapter 2 Background This chapter is divided into two parts. The first part presents a review of common SWM techniques widely practised in different countries, their advantages and disadvantages. The second part reviews low cost leachate treatment methods used for treating landfill leachates. 2.1 Solid Waste Management Techniques With a steady increase in solid waste generation, SWM has become a necessity throughout the world. Essentially, SWM deals with collection, storage and disposal of waste in an environmentally sustainable way, with minimum hazard to human health and the environment. There is no single correct method to achieve proper waste management, because management of waste has several aspects: political, social, environmental, economic and technical (Rushbrook & Finnecy, 1988). Depending on these factors, a wide range of SWM practices are prevalent in different countries. Nevertheless, this study will look at three common methods that are efficient, affordable and require minimal use of technology. 2.1.1 Composting Similar to landfills and incineration, the method of composting solid waste is nothing new. The practice of composting solid waste was prevalent over 100 years ago (Ernst, 1990; Wei et al., 2007). However, as with landfills, scientific understanding of the biological, and particularly the microbiological processes involved, has only come about recently. Until then, traditional methods of composting were largely dominant. The OM of the waste was decomposed and degraded only on a small scale under simple and natural processes and the product formed was used for agricultural land as a substitute for fertilizers. Today, composting has become an essential component of MSW management in various countries; the United Arab Emirates (UAE) – more than 50%; the USA - 33%; the UK - 15% (Farrell & Jones, 2009; Hamoda et al., 1998; Slater & Frederickson, 2001). 8 Generally, composting is described as the biological decomposition of OM under controlled aerobic conditions to form a stable, humus-like end product (Adani et al., 1995; cited in Wei et al., 2000; Farell & Jones, 2009). When MSW is involved, it is defined as a biological decomposition of the biodegradable organic fraction of municipal waste under controlled conditions, to a state sufficiently stable for nuisancefree storage, handling and for safe use in agricultural lands (Tchobanoglous & Kreith, 2002). However, in all cases, the process is facilitated by a diverse population of microbes, whose population dynamics vary greatly, both temporally and spatially, and generally involve the development of thermophilic temperatures as a result of biologically produced heat (Swan et al., 2002; cited in Farrell & Jones, 2009). Composting Procedures According to Ernst (1990) and Hamoda et al. (1998), modern composting operations consist of four basic steps: (i) preliminary treatment of feedstock, with separation of undesirable content called processing; (ii) fermentation with aeration and turning (decomposition); (iii) preparation of final product, which involves grinding, screening and final separation of undesirable items from the product; and finally (iv) marketing. The first step is necessary because the quality of compost produced will depend on the starting material. MSW is a heterogenous material containing non-compostable materials (NCM) such as heavy metals or persistent organic compounds (Lopez et al., 2010). As a result, processing of waste is required in places where there is no source segregation and sorting of waste. In the second step, fermentation/decomposition of waste begins with the establishment of composting conditions. Active involvement of microorganisms like bacteria, actinomycetes, fungi, protozoa, worms and some larvae, decompose the organic fraction, utilising it as a source of carbon. This eventually introduces an ecological succession of microbial pollutants where resident or indigenous microbes, capable of utilizing nutrients in the raw waste, immediately begin to proliferate. Due to the activity of this group, the composting mass becomes favourable for other indigenous populations to proliferate. Therefore, by plotting the effect of succession of total bacterial content of the mass would result in a curve (Figure 2.1), showing three different stages or phases in composting (Tchobanoglous & Kreith, 2002, p.12.5). 9 Finally, the third and fourth step is an essential step in determining the success of the composting and deals with the post processing and marketing of the final product. Except for the second step, the rest is common to all composting plants or industries. Types of Fermentation/Decomposition Methods To achieve fermentation or decomposition, especially for MSW composting on a commercial scale, various composting methods are employed that use systems of varying complexity. There are essentially two main types: turned or forced aeration systems. Turned systems are commonly based upon the windrow system, which entails the feedstock being piled in elongated heaps up to 2 m high and 50 m in length. Figure 2.1 Typical temperature curve observed during different compost phases (Source: Tchobanoglous & Kreith, 2002, p.12.5). These piles are turned with decreasing frequency throughout the period of active composting to maintain oxygen (O2) and moisture levels, and to release spent air. MSW windrowing is often done indoors within large commercial premises to minimize leachate production, improve odour control and reduce visual impact (Farrell & Jones, 2009). 10 A turned or windrow system sometimes consists of rotating drums responsible for receiving the waste stream. After a certain number of days, the material exits the drum and is screened. Residuals such as plastics, glass and metals are compacted and sent to landfill and organics are transferred to storage buildings for composting. Windrows are turned about two or three times per week for a total of eight weeks, during which temperatures reach 15O oC. Finished compost is screened again, prior to being sold or given away to farmers and landscapers. In contrast to turned systems, forced aeration systems are often more complex with computer controlled aeration regimes, offering a greater control over the process conditions. The Bedminster Composting Technology used by Port Stephens Council in Newcastle, Australia, is one such facility. It utilises a combination of ‘accelerated aerobic composting’ process (including a patented rotating digester system) and product screening systems to convert the organic fraction of the MSW stream into a range of high quality compost products, which are subsequently marketed. Apart from maximising the recovery of recyclable content and removing contaminants, the facility boasts achieving approximately 45% reduction in the amount of putrescible waste (Schmidt, 2005). Compost Application Compost has numerous agronomic, horticultural, and forestry uses. The largest potential user of MSW compost is the agricultural industry (Shiralipour et al., 1992). Compost that does not meet minimum environmental standards for food crop production may be used for growing nursery stock, forest seedlings, field and container grown ornamental plants (Wei et al., 2000). Low-grade composts are suitable for establishment and maintenance of public gardens and landscapes; and for reclamation of disturbed lands. Compost with excessive levels of heavy metals may be used as landfill cover or for other uses on land dedicated to disposal of waste materials. Drawbacks Even though composting is less expensive than incineration and has few negative effects on the environment, the process is a capital intensive method when compared to landfills. In addition, compost products have uncertainties attributed to pathogens, 11 heavy metals, phytotoxic compounds, and foreign objects (Rynk, 1992; cited in Wei et al., 2000). 2.1.2 Incineration Although, sanitary landfills provide a simple and affordable solution for SWM throughout the world, the need for other waste management technology does not end there. In fact, various technologies were developed in response to some fundamental flaws associated with landfills. Some of these problems were highlighted as scarcity of land, prolonged stabilisation of land filled waste and high pollution impact on the environment. The other motivation was to develop an alternative treatment tool that would allow waste reduction, material and energy recovery simultaneously within a short period of time. As a result, the period between 1970 and 2000 saw rapid development of incineration technologies (Autret et al., 2007; Joos et al., 1999; Larsen & Borrild, 1991; Yamamura, 1983). Incineration for SWM was first developed between the 1970s and 1980s in European countries, when a number of waste-energy plants were constructed. Germany, Switzerland, France, Denmark and the Netherlands chose the technology as an alternative to landfills (Autret et al., 2007; Joos et al., 1999; Larsen & Borrild, 1991). By the mid 1990s, most Asian countries (Korea, India, Taiwan and China) started to adopt incineration as an alternative to landfills (Hunsicker et al., 1996). Over time, the technology also underwent a dramatic improvement. Old incinerators with less capacity were gradually replaced with new high capacity plants, equipped with energy recovery and ash treatment facilities (Autret et al., 2007). Today, incineration is the primary means of waste disposal in several countries, including Japan (59%) and France (93%). Essentially, incineration is defined as an oxidation by controlled burning of materials to simple, mineralised products such as carbon dioxide and water. It is also described as a thermal treatment measure before the disposal of waste (Johnke, 1992). The actual combustion of waste takes place in the gas phase and simultaneously releases energy. This leads to a thermal chain reaction and self-supporting combustion, which explains why there is no need for the addition of other fuels. 12 Depending on the type of waste and its hazardous properties, incinerators are of different types with some being more elaborate and complex. The three most common incineration types are grate, rotary kiln and fluidised bed incinerators. Grate incinerators are widely used for incineration of municipal wastes while rotary and fluidised incinerators are used for hazardous wastes and pre treated wastes (sewage sludge), respectively. Grate incinerators form almost 84% of the total incinerators in France (Autret et al., 2007). Compared with landfills, incineration has several attractive features. First of all, it provides an efficient means of recovery of energy from much of the waste. For instance, the amount of heat produced from combustion is converted to steam and electricity (Baird, 1999). Secondly, it involves a large reduction in the volume to 6% and composition of the waste (Larsen & Borrild, 1991). Another major advantage is that it destroys some or all of the hazardous constituents of the solid waste and eliminates problems of methane generation and leachate production at the landfill site. Drawbacks Incineration is highly expensive and requires extensive use of technology for operation, making it less effective in terms of cost and resources. This undermines its implementation in developing countries where financial and technical resources are already a major problem. In addition, incineration of waste generates a lot of pollutants (dioxins, furans, acid gases, dust and heavy metals) along with a large amount of solid slag, called “bottom ash” (Autret et al., 2007; Mendes et al., 2004). 2.1.3 Landfills One of the main methods of disposing of municipal solid waste is to place it in a landfill, variously called a garbage dump or a rubbish dump. Initially, landfills were simply holes or a levelled piece of ground usually used for dumping municipal wastes (Al-Yaquout et al., 2002; Henry et al., 2006; Ngoc & Schnitzer, 2009; Read et al., 2001). Such landfills lacked proper design, structure and management, which led to numerous problems: pollution of surface and underground waters, unpleasant odours, pest infestation, gas explosions, public health deterioration and flooding (Ayomoh et al., 2008). 13 However, over time, landfills have undergone an immense change in structure and design due to social, technical and economic development with the purpose of ensuring minimal environmental impact (Christensen et al., 1992; Ngoc & Schnitzer, 2009). Thereby, simple and traditional landfills eventually developed into modern sanitary landfills that are now the foremost method of solid waste disposal in many countries across Asia, Africa and Europe (Renou et al., 2008). Together, traditional and modern sanitary landfills serve as the ultimate disposal destination for more than 90% of the world’s solid waste. Design and Structure The design and structure is the most important aspect of the modern sanitary landfill. Typically, the structure consists of an excavated site that has numerous “cells”, which are separate, yet connected so as to function as a single system. Figure 2.2 illustrates the desired components of a well-controlled landfill disposal facility. Figure 2.2 A sketch of a MSW landfill construction (Source: http://www.sfb477.tu-bs.de/english/tp_d1/tpd1.html). 14 Each cell is lined with a lining material used in place of, or in addition to, low permeability soils as a base or cover liner. Base liners are placed below waste to prevent leachate from making its way into the surrounding earth and ground water system, while cover liners are placed above the final waste configuration to keep water, usually rain or snow melt, from entering the waste. Although, most traditional landfills use clay or gravel for the landfill base and cover lining systems, materials such as polyethylene (PE) geo-membranes, poly vinyl chloride (PVC) and chlorinated poly ethylene (CPE) are also often used. Once the cells are ready, solid waste is deposited in them on a daily basis and periodically covered with a layer of earth or dirt, thereby establishing pockets of solid waste in the landfill (Read et al., 2001). After the landfill is filled to a predetermined amount, the site is then covered with a suitable covering material such as a layer of earth, clay or a synthetic liner to minimize leachate formation. Usually, a landfill cover with a sloping surface is preferred as it enhances surface runoff and reduces the volume of water inflow (El-Fadel et al., 1997). Leachate and Leachate Collection System As soon as the waste is disposed of in the landfill, it immediately undergoes decomposition under different conditions - aerobic and anaerobic - where it gets mixed with water, air and moisture from the waste, producing a large volume of leachate. Leachate is a highly contaminated wastewater characterised by dissolved organic and inorganic contaminants, capable of contaminating soil and water (Table 2.1). In order to reduce the potential risk to the environment and public health, leachate must be consistently monitored and controlled (Christensen et al., 2001; Duggan, 2005; Lim et al., 2009; Pivato & Gaspari, 2006). Thus, sanitary landfills are required to have systems to pump and collect leachate for further treatment. Landfills are also designed with various hydraulic barriers such as trenches and extraction and gradient control wells to make them more environmentally sustainable (Harris & Gaspar, 1989; cited in El-Fadel et al., 1997). 15 Table 2.1 Classification of Landfill Leachate According to the Composition Changes. Intermediate Stabilisation Age (Years) pH COD Type of leachate <5 <6.5 >10,000 5-10 6.5-7.5 4,00010,000 >10 >7.5 >4,000 BOD5 / COD 0.5-1.0 0.1-0.5 <0.1 Organic Compounds 80% Volatile Fatty Acids <400 <0.3 0.1-0.2 5-30% VFA + Humic and Fulvic Acids NA* 0.3-0.5 NA Humic and Fulvic Acids Low medium Low Low Important Medium Low Ammonia Nitrogen TOC/COD Total Kjeldahl Nitrogen Heavy Metal Biodegradability Young >400 >0.5 NA Note: All units in mg/L unless otherwise indicated. * Not Applicable. (Source: Foo & Hameed, 2009). Figure 2.3 shows a section of a typical leachate collection system. A leachate collection system usually consists of a network of perforated pipes located under the cover and/or above the bottom line. Each pipe is connected to a different cell, which later ends in the main system. The leachate drains are gravity fed from the landfill cells to lined leachate holding ponds (SWMC, 2002). The collected leachate is either piped to an onsite leachate storage tank for treatment or transported to an approved offsite wastewater treatment plant for disposal. In some landfills, it is recirculated in the landfill itself as a method of easier treatment and to provide water for microbial processes. Landfill Gases and Gas Collection System Apart from leachate formation, landfills pose high pollution potential owing to their ability to generate toxic gases. According to several researchers, methane and carbon dioxide are quantitatively by far the two principal components of landfill gas and form more than 90% of the total gas generated (Christensen et al., 2001, El-Fadel et al., 1997; Foo & Hameed, 2009). Nitrogen and oxygen are normally present in small quantities. Table 2.2 summarizes the composition of a typical landfill gas. 16 Figure 2.3 A cross section of a leachate collection system (Source: http://www.cintec.ca/english/03technologies/01landfills.htm). Table 2.2 Landfill Gas Composition. Concentration Range Percent Component (Dry Volume Basis) Methane 40-70 Carbon Dioxide 30-60 Carbon Monoxide 0-3 Nitrogen 3-5 Oxygen 0-3 Hydrogen 0-5 Hydrogen Sulfide 0-2 Trace Compounds 0-1 Source: El-Fadel et al. 1997, p.4. Various gases are produced due to different decomposition patterns established in the landfill. During the initial stage, waste decomposition is mainly aerobic and does not contribute to methane gas generation. OM reacts quickly with oxygen to form carbon dioxide, water and other by-products (e.g. bacterial cells). However, as oxygen is depleted within the landfill, the onset of a dominant anaerobic phase decomposition starts, which is more significant in the formation of methane gas (El-Fadel et al., 1997), as well as vapour-phase volatile organic compounds (Read et al., 2001). Apart from carbon dioxide, methane is an explosive greenhouse gas with a global warming potential estimated to be 23 times greater than that of the same volume of carbon 17 dioxide. Hence, release of methane and carbon dioxide from the landfill surfaces gives rise to greenhouse gases (El-Fadel et al., 1997; Themelis & Ulloa, 2007). Based on this determination, it is apparent that landfill gas control and recovery measures are essential in eliminating or minimizing environmental impacts. To efficiently control gas and avoid odour problems, gas extraction systems may require installation of larger pipes, blowers and related equipment early in the landfill’s operational life. Horizontal trenches, vertical wells, near surface collectors, or a hybrid system may be used for gas extraction. Typically, landfills should have one well per acre (0.4 ha) but greater gas flows can also be readily accommodated by increased pipe diameter. An example of a landfill gas collection and recovery system is shown in Figure 2.4. With the help of an extractor fan to pull the gas from the collection wells, landfill gases are then piped to a main collection header where they are treated or flared. Figure 2.4 A landfill gas collection and recovery system (Source: Tchobanoglous & Kreith, 2002, p.14.7). 18 2.2 Review of Leachate Treatment Options Historically, most landfills rely on the slow uncontrolled release of leachate into the surrounding geology and ground water or surface water (Duggan, 2005). While many such cases have been superseded by modern sanitary landfills and better technologies, much still needs to be done in order to reduce contamination of the environment. As a result, treatment of leachate is still a priority for the waste industry. A treatment method is referred to as any method capable of reducing the concentration of the pollutants in the leachate to a level considered safe for the environment and human health. Currently, a number of options are available for leachate treatment depending upon the nature and strength of leachate and the degree of treatment required. These treatment options are based on physical, chemical, biological or a combination of one or two of these processes. 2.2.1 Aerobic biological treatment (Aeration) Definition One of the simplest forms of on-site treatment of landfill leachate is by means of aeration. A typical aerated lagoon or pond refers to basins constructed in, or on the ground surface, using earthen dikes to retain wastewater, within which natural stabilization processes occur with the help of natural or artificial aeration. Because, most landfill leachates are characterised by high concentrations of OM and ammoniacal nitrogen, this method is chosen particularly to solve this problem with minimum cost incurred. It is therefore important to remember that most often, the method may not achieve the best effluent quality technically possible, but will still provide the effluent to meet discharge consent conditions, while minimising the total cost of onsite treatment. The Concept of Aeration The principle of aeration is that it can bring about changes in the leachate via chemical and biological oxidation, thereby allowing its quality to improve (Mehmood et al., 2009; Nivala et al., 2007; Robinson & Grantham, 1988). The concept of supplying oxygen to the leachate is to bring about aerobic degradation of organic material and subsequently transform the nitrogen content of the leachate. As aeration continues, the 19 heterogeneous mass of new cells synthesized is further destroyed due to endogenous respiration/self oxidation in order to yield the required energy. This happens in all the aerobic treatment systems such as the aerobic stabilisation pond, oxidation ditches and aerated lagoons. Once the carbonaceous demand is satisfied, oxidation of nitrogenous material occurs via nitrification. Nitrification occurs because leachates usually contain nitrogen compounds in the form of ammonia (NH3), nitrite (NO2-), nitrate (NO3-), amines and other nitrogenated compounds. Nitrification occurs in a two-step aerobic biological process. Firstly, ammonia is converted to nitrite by Nitrosomonas and thereafter, nitrite is quickly converted to nitrate through the action of Nitrobacter. The process of nitrification is influenced by several factors: dissolved oxygen (DO); temperature; pH; total alkalinity; and detention time. Classification of Aerobic Treatment of Leachate There are three types of aerobic treatment based on the way total solids in the system are handled: i) Facultative type, ii) Aerobic flow-through type, iii) Extended aeration type. Facultative Lagoons Facultative lagoons are akin to algal ponds used for waste stabilisation except that the O2 is derived from mechanical aeration instead of algal photosynthesis. In this kind of pond, the power input is only sufficient to diffuse enough oxygen into the liquid and not to maintain all the solids in suspension. Consequently, suspended solids in the raw sewage entering the lagoon tend to settle down and undergo anaerobic decomposition at the bottom. The activity in such a lagoon is therefore partly aerobic and partly anaerobic, which contributes to the name “facultative” (Arceivala, 1973). Facultative ponds in the form of onsite-aerated lagoons are so far the most common method of treating wastewater in developing countries. Researchers have identified the method as a promising technology among others because of its extreme simplicity, low cost and minimum usage of technology (Mehmood et al., 2009; Robinson & Grantham, 1988). 20 Such lagoons can achieve good BOD removals and are mostly used in the treatment of sewage, industrial wastes and leachates. Frascari et al. (2004) and Mehmood et al. (2009) reported on the successful facultative lagoon treatment used for treating landfill leachate in Italy and the United Kingdom (UK), respectively. The facultative lagoon achieved significant removal efficiency of 64%, 40%, 77%, 77% and 63% for BOD, COD, ammonia, Total Kjeldahl Nitrogen (TKN), and nitrate, respectively. In the UK, facultative aerobic systems involving sequential aerobic and anaerobic microbial oxidations achieved 75% COD and 81% ammonia removal, respectively, at a higher hydraulic retention time of 56 days. Aerobic Flow-through Type Similar to the facultative lagoons, the aerobic flow-through uses oxygen or air and microbial action to bio-treat the pollutants in wastewaters. However, it makes extensive use of energy to keep all the solids in suspension, ensuring complete mixing conditions. Another different feature is that this method does not hold back or retain solids. Instead, solids are allowed to pass out along with the effluent. Consequently, the BOD removal efficiency is not very high. An example of the efficacy of a flow-through lagoon was assessed by Robinson & Maris (1983) in a bench scale experiment. Apart from demonstrating a good removal of BOD (97.5%) and COD (92%) at mean solid retention time (SRT) of 10 days, the study exhibited the possibility of metal removal via the process of aeration. Removal efficiency for various metals was recorded as: iron (Fe) (>98%); manganese (Mn) (>92%); and zinc (Zn) (94%). Extended Aeration Lagoons This type of lagoon is similar to the aerobic flow through type in the sense that it also maintains solids in suspension and complete mixing conditions are attained. However solids are not allowed to flow out with the effluent, rather, they are deliberately made to accumulate or build-up in the system, either by providing a separate settling tank or recirculation, or by incorporation of a settling compartment in the lagoon itself. Similar methods of biological processes for treatment of wastewaters are also prevalent in various hybrids but all the methods have in common the use of air and microbial action to bio-treat the pollutants in wastewater. 21 Design and Operation Aerated lagoons are generally rectangular in shape and are three to five metres in depth and built in earthwork with slopes partly or fully pitched in stone or concrete. Figure 2.5 below shows an example of an aerated lagoon built in an concrete structure. The inlet and outlet are located on opposite banks. To avoid percolation, a lagoon has to be located in a relatively impervious soil or suitably lined or constructed in masonry and concrete and in most cases lined with high-density chlorinated polyethylene materials. Leachate is collected by differing methods including pneumatic pumps, hydraulic pumps and standard electric pumps. In situations where the landfill is raised above the land level, it is also possible to use gravity to collect and move leachate at a lesser cost. One very important feature of an aeration plant is the provision of efficient and proper diffusion of air. Usually, a range of methods are available - surface aerators, venturi injectors and air diffusers - but a spiral flow system, which uses an air diffuser is considered more convenient and advantageous because a single air header can serve two tanks with diffusers located along one wall of the tank. It also allows fine bubble diffused aeration, which helps in providing a high level of oxygen transfer and is very gentle with bacteria. Another less expensive method of aeration is to use floating aerators and air from atmospheric diffusion and photosynthetic sources. Figure 2.5 An aerated lagoon plant (Source: http://www.adadget.com/domestic.html). 22 2.2.2 Flocculation Coagulation/flocculation is a relatively simple technique that may be employed successfully for the treatment of stabilised or older landfill leachates and wastewater. It involves physio-chemical processes considered effective for removing metals, turbidity and refractory humic substances (Tatsi et al., 2003; Li et al., 2010). Quite often, flocculation is combined with precipitation and sedimentation in removing metals from leachate. Precipitation involves the addition of chemicals to the leachate to transform dissolved contaminants into insoluble precipitates, followed by flocculation and then finally, removed by sedimentation or filtration (McArdle et al., 1988). The precipitation method is necessary in flocculation because it allows metals to precipitate from leachates as hydroxides, sulphides, or carbonates by the addition of an appropriate chemical precipitant and the adjustment of its pH to favour solubility. Although better removal efficiencies are possible with sulphide precipitation, hydroxide precipitation with lime or caustic as the precipitant is practised widely because of the materialshandling and cost advantages (Canter & Knox 1986; cited in McArdle et al., 1988). Principle of Flocculation In the wastewater treatment industry, the first step in the coagulation-flocculation method is to destabilise the dispersion and coagulate the contaminants. This is generally done via the addition of positively charged species, called as coagulants, in appropriate quantities to neutralise the charge on the impurities. A flocculation step is then used to bring together the small flocs formed by coagulation so that larger flocs that will precipitate are produced. Coagulants form an essential part of the coagulation-flocculation treatment, thus it is important to know the various coagulants used. The most widely used coagulants are salts of aluminium (Al) and iron (Fe) such as aluminium sulphate, ferrous sulphate and chlorides and polyaluminium chloride (Amokrane et al., 1997; Aziz et al., 2007; Li et al., 2010; Ntampou et al., 2006). While Al and Fe salts have been widely used for removing humic substances from water, ferric chloride is suggested as a viable coagulant in managing colour problems in leachate treatment. Polyaluminium silicate chloride is a new coagulant reagent, which has enhanced aggregating power and the 23 ability to form bigger and denser floc formation (Tzoupanos et al., 2008). Calcium hydroxide (lime) is used most of the time for precipitation. Drawbacks Flocculation firstly allows only moderate removal of COD (or TOC) content. Secondly, the process results in production of excessive sludge and increased aluminium (Al) or iron (Fe) concentrations in the resulting effluent (Maranon et al., 2008). As a result, coagulation-flocculation has been proposed mainly as a pre-treatment method for fresh leachates prior to biological, physical or other chemical techniques or as a posttreatment technique for partially stabilized leachate (Tzoupanos et al., 2008). 2.2.3 Membrane separation Membrane separation is based on the principle that semi-permeable membranes allow only water and certain solutes to pass through them. Essentially, two processes are involved: ultrafiltration and reverse osmosis. Based on a similar concept, granular-media filtration uses a bed of granular material to remove the suspended solids from leachate by forcing the fluid through this porous medium. The granular media filtration system may be classified by: 1) direction of flow, 2) type of filter beds, 3) waste water driving force, and the method of flow rate control. For high turbidity leachate, dual or tri-media filter beds are more desirable because they have greater solids storage capacity. 2.2.4 Activated Carbon Adsorption (ACA) A notable trend in the development of activated adsorption was seen over the last few years in wastewater treatment industries. Activated Carbon has a superior ability for removal of a wide variety of organic and inorganic pollutants dissolved in aqueous media (Foo & Hameed, 2009) due to its large porous surface area, controllable pore structure, thermo stability and low acid or base reactivity. Gradually, activated carbon is used as an adsorbent for separation of marginally biodegradable organic and highly toxic inorganic micro pollutants (heavy metals) (Christensen et al., 1992). Thus, it has greater applications in leachates containing non biodegradable substrates, which are not 24 removed by biological treatment alone (Cecen & Cakiroglu, 2001; cited in Cecen et al., 2003). Apart from granular activated carbon (GAC), powdered activated carbon (PAC) is also used in water treatment. Cecen & Aktas (2001) found that PAC is suitable in leachate treatment based on the ability to enhance biological treatment efficiency and nitrification by completely preventing nitrification inhibition. PAC, when combined with activated sludge, improves sludge dewater ability and increases the removal efficiency by adsorbing non-biodegradable, toxic or inhibitory organics and also some metals (Metcalf & Eddy, 2004 ; cited in Aghamohammadi et al., 2007). The advantages of using activated carbon adsorption in the passive leachate treatment industry over the last 15 years are reflected in several studies (Foo & Hameed, 2009; Kargi & Pamukoglu, 2004) The studies illustrated the use of GAC and PAC as a successful treatment option with 91% and 95% COD removal, respectively. Recently, GAC, granular activated alumina and ferric chloride were utilised for the treatment of heavy metals (Cd, Cu, Cr, Mn, Pb and Zn). Foo & Hameed (2009) studied the use of GAC and limestone in removal of ammonium nitrogen. 25 Chapter 3 Solid Waste Management This chapter compares in brief the waste management system in Bhutan to that of Summerhill Waste Management Centre (SWMC) in Newcastle. An overview of the main physio-chemical characteristics of leachate formed at the SWMC sanitary landfill during the past 14 years is presented. In addition, the outcomes of the statistical evaluation of long term leachate monitoring data set are discussed in relation to leachate formation at SWMC sanitary landfill. The correlations between the volume of leachate generated and the rainfall received at the site are applied in estimating leachate formation at SWMC sanitary landfill and Bhutan. 3.1 Waste Management in Bhutan As in other countries, waste management in Bhutan consists of two main steps: In the first step, collection and transportation of waste is carried from house-tohouse at regular intervals by employing trucks/tippers. The recent trend in urban centres is towards using concrete receptacles and bins placed at strategic points from where the garbage is lifted for removal by trucks or tractors (State of the Environment, Bhutan, 2001). However, all the three components of waste management - collection, transportation and disposal - lack in terms of infrastructure, maintenance and up gradation. The second step deals with disposal of waste in the landfills. In the absence of alternative waste management methods, 90% of the MSW is disposed of in the landfills. Generally, each urban centre has only one or no landfill. These landfills receive the entire MSW. Where there is no landfill site, MSW is disposed of either in rivers/streams, valleys or in low lying areas. About 50-60% of the MSW disposed in the landfill is organic in nature, which is an indication that MSW contains very little glass, electronics and metals (Phuntsho et al., 2008). The overall composition of the MSW waste in Bhutan is shown in Figure 3.1. 26 Figure 3.1 Average percentage composition of MSW in Bhutan (Source: Phuntsho et al., 2008). 3.1.1 Problems of SWM in Bhutan In many regions, the solid waste problems are becoming acute. New sites that are both accessible and technically suitable for landfills are almost impossible to obtain, due to lack of vacant land, finance and labour. Currently, Memelakha Waste Disposal Site in Thimphu is the largest landfill in the country. It is located 12 km away from the city and receives about 64.5 tonnes of MSW daily. Collection of solid wastes, transportation and disposal at the landfill site was initiated in 1993. However, the landfill has exceeded its volumetric capacity of 70,000-80,000 m3 for years and is already overflowing with wastes. Yet no alternative site has been identified (Allison, 2008; Penjor, 2008). Furthermore, the landfill does not have adequate leachate and gas collection and treatment systems. Likewise, there are already serious concerns about the sustainability and management of landfills in several other cities. Concerns centre on three main features: (i) increasing population and waste output (ii) lack of land to identify a landfill and (iii) pollution potential of the landfill leachate and gases. Apart from the problem of disposal, the worst problem of waste management in Bhutan is the lack of proper segregation, sorting and recycling of waste (Phuntsho et al., 2008). 27 Proper segregation of waste into different components and their separate collection do not occur at source. Thus, the entire organic and inorganic wastes are disposed of in the landfills. 3.2 Summerhill Waste Management Centre (SWMC), Newcastle Newcastle is the second most populated area in the Australian state of New South Wales. It is situated 162 kilometres northeast of Sydney on the southern bank of the Hunter River. According to the 2006 census the city has a population of 288,732 persons, which is equivalent to a population of several cities in Bhutan and one third of population of Bhutan. SWMC in Newcastle is a high-tech, high capital cost facility with many state-of-the-art aspects of waste management (Whitehead et al., 2000). Located on the site of a former open-cut and underground coal mine, which includes tracts of remnant vegetation and areas disturbed by disused workings, the site is close to Wentworth Creek and Flaggy Creek catchments (SWMC, 2002). It is a class one solid waste landfill managed by the City of Newcastle and has a total approved capacity of 3.5 million m3 (Whitehead et al., 2000). Since 1995, some 150,000 tonnes of waste has been received at the site annually. The waste received by the landfill includes both putrescible and non-putrescible waste. However, unlike in Bhutan, where MSW mainly originates from residential sources (69%) (Penjor, 2008), the majority of the waste received by the Summerhill landfill originates primarily from commercial and industrial sites in Newcastle. A smaller portion of the waste comes from public or council operations. For example, in 2001 and 2002, 55% of its waste received by the landfill was produced by commercial and industrial sites; 29% household and 16% by the public or from council operations. Solid waste containing large proportions of putrescibles waste constitutes the major portion (52%) of the waste stream (SWMC, 2002). This indicates that about 50% of the waste received at the centre undergoes compaction, decomposition and thus, is liable for leachate production in the long run. 28 Unlike landfills in Bhutan, the special feature of the SWMC landfill is that it comprises a series of discrete sections called “cells” where different wastes can be processed and deposited. The cells are constructed with a foundation layer of between 0.5 and 2.0 metres of onsite clay over a prepared subgrade. It is then lined by manufactured geocomposite clay and a high density polyethylene (HDPE) synthetic liner. The HDPE liner functions as an impermeable barrier to leachate and helps in collection and reduction of leachate generation, thereby minimising the contamination of groundwater and downstream surface water (Whitehead et al., 2000). In contrast to disposal of waste in Bhutan, SWMC has a special method of disposing waste in the cells. Waste is deposited and compacted in successive layers called “lifts” and covered daily with approximately 150 mm of soil in order to minimise and control water infiltration, odour, flies, litter, fire and vermin. Finally, the cells are covered with 600 mm of compacted low permeability clay cover, 1250 mm of bulk capping, topsoil and vegetation (Whitehead et al., 2000). On top of that, the landfill site has been designed to carry out various functions necessary for proper management of waste. The design consists of a computerised weigh bridge system, waste segregation and recycling facilities. The centre also has an administrative, exhibition and educational facilities on waste minimisation and recycling activities. Other provisions of the centre include comprehensive monitoring of leachate, surface and groundwater, noise, dust, odour and litter (SWMC, 2002; Whitehead et al., 2000). The landfill sites in Bhutan do not have such facilities. While Bhutan does not have a pricing mechanism imposed on waste disposal, SWMC imposes a levy of $65.30 per tonne on all waste disposed of at the centre. The imposition of landfill levy is in accordance with New South Wale Government’s Waste Minimisation and Management Act 1995, which set an ambitious target of a 60% reduction in waste to landfill by 2000 (Oakes, 2009). 29 3.3 Leachate Characterization and Statistical Evaluation The characteristics of a typical leachate generated at the SWMC landfill have been investigated by analysis of long-term leachate monitoring data collected from the centre. The main purpose of this analysis was to understand various aspects of a landfill (sanitary), particularly, the pollution potential associated with leachate formation. Apart from this, the investigation was expected to establish, if any, the correlation of leachate characteristics to some important climatic factors (rainfall), which might be useful in considering leachate characteristics and leachate generation in Bhutan. Table 3.1 presents a summary of the main physio-chemical characteristics of the leachate monitored during the past 14 years by SWMC. The different water quality parameters are divided into three different groups and the values are calculated by various preliminary statistical treatments, i.e., calculation of average (mean) and range (minimum to maximum). A wide variation is observed in the quality of leachate. Overall, the organic content of the leachate exhibited very high variations followed by inorganic macro components. Trace elements were found only in traces and showed much lesser variation. Physical pollution parameters such as pH, EC, total alkalinity, and suspended solids also showed frequent variations. The organic content of the leachate are indicated by various parameters (BOD, COD and TOC) in the range (min-max) 2-2750 mg/L, 80-3850 mg/L, 1-1840 mg/L, respectively. In terms of inorganic macro components, ammonia-nitrogen is the most significant pollutant with concentration in the range 0.01-970 mg/L. With the exception of nitritenitrogen (0.01-0.50 mg/L), nitrate-nitrogen (0.01-0.2 mg/L), phosphate (0.10 mg/L) and Manganese (Mn) (0.2-3.0 mg/L), metal salts such as sodium (Na) and potassium (K) were also present in high concentrations (50-2160 mg/L and 5-900 mg/L, respectively). Chloride and sulphate varied from 30-2450 mg/L and 1-400 mg/L, respectively. 30 Table 3.1 Composition of Landfill Leachate (1995-2009) from SWMC, Newcastle, Australia. pH No. of sample 188 Range 6-8 Mean 7.8 Electrical Conductivity (µs/cm) 157 105016500 6250 59 170 76-6950 10-7220 1600 337 187 2-2750 306 180 178 80-3850 1-1850 944 309 189 37 0.01-970 0.01-0.50 200 0.10 78 1 78 84 78 78 77 78 56 46 0.01-2.00 0.10 30-2450 1-400 50-2160 5- 900 2-300 30-250 0.01-430 0.2-3.0 0.10 0.10 728 85 597 217 86 118 19 1.0 42 79 9 0.00-0.05 0.00-0.20 0.01-0.07 0.01 0.05 0.03 Parameter Total Alkalinity (as mg/L as CaCO3) Suspended Solids (NFR) Organic Matter Biochemical Oxygen Demand (BOD5) Chemical Oxygen Demand (COD) Total Organic Carbon (TOC) Inorganic macro components Ammonia-nitrogen Nitrite-nitrogen Nitrate-nitrogen Phosphate Chloride Sulphate Sodium Potassium Calcium Magnesium Iron Manganese Inorganic trace elements Cadmium Chromium Cobalt Copper Lead Mercury Nickel Zinc 0.0076 18.00 0.50 43 0.00-0.10 0.04 17 0.00-0.08 0.01 74 0.01-0.4 0.10 45 0.01-0.1 0.20 Note: All units in mg/L unless otherwise indicated. 31 Compared to Na and K, the concentrations of other metal salts are comparatively low and lay in the range 2-280 mg/L for calcium (Ca), 30-250 mg/L for magnesium (Mg) and 0.01-430 mg/L for iron (Fe). The high concentration of inorganic components might have led to the high values of conductivity, total alkalinity and suspended solids. On the other hand, a large number of xenobiotic organic compounds (XOCs) (aromatic hydrocarbon, phenols, chlorinated aliphatic compounds and pesticides) and heavy metals (cadmium (Cd), chromium (Cr), cobalt (Co), lead (Pb), mercury (Hg), nickel (Ni) and zinc (Zn)) were present only in traces with most of their concentrations remaining below 0.5 mg/L. The Australian Water Quality Guidelines for Fresh and Marine Waters (AWQGFMW) (1992), considered 0.5 mg/L or lower as a limit value safe for fresh and marine waters. Further detail of some of this important parameter with increasing age of the landfill is discussed in the section below. 3.3.1 Temporal variation in leachate quality The analysis of the leachate composition data for the past 14 years indicated that temporal variation in leachate quality is evident at the SWMC landfill. Figures a-g in Appendix B-1 illustrate the temporal variations in some of the main pollutant parameters of the leachate as a function of time (landfill age) that extended over a period of 14 years (1995-2009). The parameters are ammonia-nitrogen, pH, EC, suspended solids, BOD, TOC, COD, metals and salts. The respective curves clearly show that the landfill underwent two distinct decomposition phases with increasing age. The first phase (1995-1999) resulted in production of weaker or less toxic leachate, while the latter (2000-2009) recorded the production of higher strength leachate. The First Phase - from 1995 - 1999 The first phase of the landfill included an initial stabilisation phase that lasted for approximately four to five years. Leachate collected during this phase was a result of first decomposition activity taking place within the landfill. Mean concentrations of the resulting leachate shows that the leachate produced during the initial period is less toxic with a fairly neutral pH (mean value ~7) and low concentrations of ammonia-nitrogen (6 mg/L), nitrite-nitrogen (0.01 mg/L), nitrate-nitrogen (0.03 mg/L), Na (120 mg/L), K (3 mg/L) and Fe (1 mg/L). Both chloride and sulphate are approximately estimated as 32 130 mg/L. Trace elements have even lower concentration of less than 1 mg/L. Total alkalinity measured 580 mg/L asCaCO3 and EC value was 850 µS/cm. The organic compounds in the leachate were detected only towards the end of the first year as indicated by high BOD (900 mg/L) and COD (1300 mg/L). The Second Phase - from 2000-2009 Unlike the first phase, the second phase of landfill was marked by a significant change in the quality of leachate, indicating a gradual change in its decomposition pattern. The phase was marked by an increasing concentration of ammonia-nitrogen, chloride, sulphate and Na and K. The leachate became slightly alkaline along with an increase in EC and alkalinity. This assumption is in agreement with Chen (1996), who reported that an increasing age of landfill led to an increase in pH value up to a certain steady state. In the case of high EC, the author attributes this to the increasingly dissolved solids present in the leachate. The changing pattern of the BOD and COD are in agreement with the changes observed in other landfills i.e., BOD decreased with time while COD kept increasing and that COD is higher than BOD and TOC. Of the nitrogenous compounds, ammonia-nitrogen increased rapidly owing to the deamination of amino acids present in the organic waste (Tatsi & Zoubolis, 2002). Since the concentrations of nitrite-nitrogen and nitratenitrogen remained constant without much change, ammonia nitrogen is the major portion of total nitrogen present during this phase, hence an anaerobic state. However, more changes were observed with increasing age. Leachates became more concentrated and hence more toxic. In addition to ammonia, the landfill also produced methane gas. A routine surface gas monitoring in 2002 detected three locations where methane gas levels were greater than 1% Lower Explosive Limit (LEL) (SWMC, 2002). In addition to the normal decomposition changes that are described above, highly concentrated leachates were produced between 2001 and 2003. During this period, leachates showed an unusual increase in some pollutant parameters, particularly with respect to ammonia, OM and salinity. Therefore, a pollutant peak can be observed in this period in the Figures a-g in Appendix B-1. This is related to a reduction in dilution 33 caused by the comparatively lower amount of rainfall (900 mm/year) received in this period compared to the earlier period (1000 mm/year). Christensen et al. (2001) observed similar changes in other landfills as well. 3.4 Outcomes of Leachate Characterization and Evaluation The characterization of long term leachate data set resulted in three significant outcomes: i) Formation of SWMC landfill leachate and its toxicity, ii) The relation between climatic conditions (rainfall) and leachate formation at the SWMC landfill site, iii) Prediction of leachate formation in Bhutan. Firstly, the analysis indicated the possible time of leachate formation and the main pollutant parameters of the SWMC landfill leachate. That is leachate formation at this landfill site occurred as early as the first year of landfill operation in 1995. Hence, researchers have suggested leachate formation as one principal cause of environmental risks associated with landfills (Christensen et al., 2001; Duggan, 2005; Lim et al., 2009; Pivato & Gaspari, 2006; Tatsi & Zoubolis, 2002). Ammonia-nitrogen was the main pollution potential of the SWMC landfill leachate between 1995 and 2009. Although Figure 3.2 revealed that high concentrations of ammonia-nitrogen occurred only rarely, its mean concentration remained approximately >200 mg/L, which is much higher than the 0.1-2.5 mg/L limit recommended by AWQGFMW (1992). Thus, it is clear that ammonia can have negative environmental impact through toxicity. Leachates of this nature, if released directly into the surrounding environment, can elevate ammonia concentrations in fresh waters by almost 100 times making it unsafe for fresh water aquatic organisms. Above 0.1-2.5 mg/L, ammonia can cause acute and chronic toxicity to fresh water fish (AWQGFMW (1992). Apart from ammonia-nitrogen, the high concentration of Fe (mean 19 mg/L or 19000 ug/L), is likely to make the ammonia in the leachate more toxic. Warnock & Bell (1969) 34 in AWQGFMW (1992) reported that Fe concentration between 320 µg/l and 16,000 µg/l can be toxic to aquatic insects. Fresh water bodies are considered safe only at Fe concentration of 1000 µg/l or 1 mg/L. Figure 3.2 Frequency distribution curve of ammonia between 1995 and 2009. Secondly, the study of leachate formation at the SWMC site under varying rainfall conditions [ i) monthly leachate production (2001-2009) against monthly rainfall (mm) (Figure 3.3) and ii) annual leachate production (1995-2009) against annual rainfall (mm) (Figure 3.4)] revealed that leachate percolation is closely correlated with rainfall during the monitoring period. The seasonal variation in leachate formation is evident from the fact that the summer season with higher rainfall resulted in higher volumes of leachate. Climatic conditions (mean annual) during summer are generally characterized by higher rainfall, temperatures and relative humidity compared to winter. Similarly, the annual leachate production was roughly proportional to the amount of annual rainfall received in each year of the monitoring record. Further confirmations of this relationship have been derived using linear (Figure 3.5a, b) and bivariate correlations (Table 3.2) between the rainfall and leachate formation. The results supported the hypothesis that a positive relationship exists between the two variables and indicates that higher volumes of leachate generated are associated with higher levels of rainfall (r=0.488 and 0.606, p<0.05). 35 Figure 3.3 An illustration of six month moving average – leachate percolation versus rainfall (January 2001- December 2009). 36 Figure 3.4 An illustration of the annual leachate percolation versus annual rainfall received at the landfill site (19952009). 37 a b Figure 3.5 A linear relationship between rainfall (mm) and leachate volume (KL): a) monthly (R2= 0.238) and b) annual (R2= 0.367). 38 Table 3.2 Correlations Between Rainfall in mm and Leachate Volume in KL: i) monthly (2001-2009) and ii) annual (1995 -2009). Monthly Leachate Monthly Volume (KL) Rainfall (mm) Monthly Leachate Volume (KL) Pearson Correlation Sig. (1tailed) N 1 0.488** 108 Annual Leachate Volume (KL) 0.000 108 Annual Rainfall (mm) Pearson 1 0.606** Correlation Sig. (1Annual Leachate 0.008 tailed) Volume (KL) N 15 15 Note: ** Correlation is significant at the 0.01 level (1-tailed). Similar relations between climatic conditions and the amount of leachate production in different climatic zones have been reported by several researchers (Frascari et al., 2004; Nora, 2007; Robinson & Grantham, 1988; Tatsi & Zoubolis, 2002). While Frascari et al. (2004) confirmed close relations between rainfall and leachate production at an active Italian landfill site in north Italy, Tatsi & Zoubolis (2002) reported that Mediterranean climates with dry, hot seasons resulted in reduced percolation of leachate at a landfill in Thessaloniki, Greece. Further evidence of the effect of rainfall on leachate production is observed in countries like Thailand and Malaysia, which have extreme climates characterized by high intensity rainfall (up to 80 mm/day and above) in the rainy season and no rainfall at all in the dry season (Nora, 2007). Finally, considering the fact that rainfall is one of the most significant factors responsible for leachate formation at any landfill site, landfills in Bhutan are likely to generate very high volumes of leachate as compared to other countries because the majority of the urban settlement areas are located in areas that receive more rainfall (500-5000 mm per year) (Table 3.3). 39 Table 3.3 Spatial and Altitudinal Variation in Annual Rainfall by Region and Percentage of Total Area in Different Altitudinal Zones in Bhutan. Altitude(m) % Area Region Below 600 5.3 600-1800 22.4 1800-4000 51.8 Above 4000m elevation 20.5 Southern border area Southern foothills Inner central valleys /Himalayas Greater Himalayas Annual rainfall (mm) 30005000mm 12002000mm 5001000mm Less than 500mm Source: Country Report on the Forest Resources of Bhutan, 2000. Unlike Australia, the monsoon or summer season in Bhutan falls between June and August; therefore, higher leachate percolation could occur during this period. The other implication is that landfill sites situated in different altitudinal zones will have different leachate percolation because the amount of rainfall received decreases with the increase in altitude, as shown in Figure 3.6. Figure 3.6 A plot of variation in rainfall with changes in season and altitude in Bhutan. 40 Chapter 4 Materials and Methods This chapter describes the two elements of the research undertaken. It includes procedures undertaken to study two bench scale laboratory experiments and leachate characterisation from long term monitoring of data collected at the SWMC, Newcastle. The objective of this element of the research was to evaluate the suitability of two passive leachate treatment options, which are simple, effective and affordable. In order to achieve this, two independent bench scale experiments were conducted in the laboratory - (1) a passive aeration of leachate in 25 litre tanks and (2) column experiments using three different filter materials (Granular Activated Carbon (GAC), Blast Furnace Slag (BFS) and sand). The laboratory scale experiments were undertaken to determine whether the results can be applied to or assist with the design of larger scale treatment methods suitable for treatment of landfill leachate in Bhutan. The procedures associated with each of the experiments are described below. 4.1 Aerobic Biological Treatment 4.1.1 Site selection Figure 4.1 shows the modern sanitary landfill at SWMC, Newcastle, NSW. The background of the landfill is described earlier in Section 3.2.. General climatic conditions between 1995 and 2009 include mean rainfall and temperature of 1165 mm/year and 24 oC, respectively, 70% humidity and minimum mean sunshine of 7 hours. The climate data were obtained from the Williamtown meteorological station (site number 061078), which is located approximately 16 km from the landfill site at an elevation of 9 m above sea level (Bureau of Meteorology (BOM), 2010). 4.1.2 Physical and chemical parameters of the leachate (sample) Nine different water quality parameters were examined as part of this experiment: turbidity and colour; pH; total alkalinity; electrical conductivity; ammonia-nitrogen; nitrite-nitrogen; nitrate-nitrogen;and SRP (SRP). Additional factors such as DO and 41 temperature were also examined in this experiment. All the methods are based on Standard Methods for the Examination of Water and Wastewater (Eaton et al., 1995). The solid waste disposal area. Figure 4.1 SWMC landfill and methane collection facility. Turbidity and colour Turbidity of the waste water indicates the “clarity” of the sample and is an important determinant of its condition and productivity. Also defined as the expression of the optical property that causes light to be scattered and absorbed, turbidity is caused by suspended and colloidal matter such as clay, silt, finely divided organic and inorganic matter, plankton and other microscopic organisms. The higher the intensity of scattered light, higher the turbidity. In this study, turbidity was determined by the Absorptometric method using a HACH turbidity meter 2100P. In addition to turbidity, colour was qualitatively assessed. 42 Acidity (pH) and Total Alkalinity While pH indicates the acidity of water or a solution, total alkalinity of water is its acid neutralising capacity. Total alkalinity indicates the buffer capacity of the water, that is, its ability to resist small additions of acids to change pH. It is expressed as mg/L as calcium carbonate (CaCO3) and indicates the concentration of carbonate, bicarbonate and hydroxide contents of the fresh water. The measured values may also include contributions from borates, phosphates, silicates or other bases, if present. Alkalinity is primarily used in interpretation and control of water and wastewater treatment processes. In this study, total alkalinity of the leachate samples was determined by HACH Alkalinity test kit digital titrator-16900 (model-AL-DT) for alkalinity values in the range 10 - 4000 mg/L as CaCO3. pH was determined by a pH meter connected to a HQ 40d DO probe. Electrical conductivity (EC) EC is a measure of the ability of an aqueous solution to carry an electric current. This ability depends on the presence of ions; on their total concentration, mobility, and valence; and on the temperature of measurement. Solutions of most inorganic compounds are relatively good conductors. EC was determined by the conductivity meter attached to a HQ 40d DO probe. The EC electrode was first calibrated to EC of 1412 µS/cm before determining the EC. Inorganic Constituents (Nitrogen compounds and Phosphorus) Ammonia-nitrogen (NH3-N) is produced largely by deamination of organic nitrogen and by hydrolysis of urea. It is present naturally in surface and waste waters. Ammonia concentrations encountered in water vary from >10 ug/L in some natural surface and ground waters to more than 30 mg/L in some wastewaters. In the laboratory, NH3-N is determined by the Phenate Method, which is based on the principle that an intensely blue compound, indophenol, is formed by the reaction of NH3, hypochlorite, and phenol catalysed by sodium nitroprusside. The method used HACH TNT 832 test reagent capable of determining high range NH3-N in the range 2-47 mg/L. Nitrite-nitrogen (NO2--N) is an intermediate oxidation state of nitrogen both in the oxidation of ammonia to nitrate and in the reduction of nitrate. Such oxidation and 43 reductions may occur in waste water treatment plants, water distribution systems and natural waters. NO2--N 2 - is the actual etiologic agent of methemoglobinemia. NO2--N is determined by HACH TNT 839 test reagent, which can determine NO2--N - concentration in the range 0.015-0.60 mg/L and 0.5-2.00 mg/L. The test is based on a principle that nitrites react with primary aromatic amines in acidic solution to form diazonium salts. These combine with aromatic compounds that contain an amino group or hydroxyl group to form intensively coloured azo dyes. Nitrate-nitrogen (NO3--N) forms part of the nitrogen cycle and generally occurs in trace quantities in surface water but may attain high levels in some groundwater. In excessive amounts, it is considered to contribute to methemoglobinemia in infants. A limit of 10 mg/L NO3--N has been thus imposed on drinking water to prevent this condition. NO3-N is determined using high range (5-35 mg/L and 22-155 mg/L) HACH TNT 836 test reagent based on the principle that NO3--N ions in solute ions containing sulphuric acid and phosphoric acids react with 2, 6-dimmethylphenol to form 4-nitro-2, 6dimethylphenol. Soluble Reactive Phosphorus (SRP) occurs in natural waters and in waste waters almost solely as phosphates in the form of particles or detritus, or in the bodies of aquatic organisms. These are classified as SRP, condensed phosphates and organically bound phosphates. They arise from a variety of sources such as surface run off or drainage water. The main concern related with phosphorus is the eutrophication of surface waters. Phosphorus determination in this experiment used the principle that phosphate ions react with molybdate and antimony ions in an acidic solution to form an antimonyl phosphor molybdate complex, which is reduced by ascorbic acid to phospho molybdenum blue. HACH TNT 843 test reagent for determining low range SRP (0.051.50 and 0.15-4.50 mg/L phosphorus was used for the test. All the inorganic constituents were measured by HACH DR2800 Spectrophotometer. 4.1.3 Characterisation of leachate sample Raw landfill leachate was collected from the leachate pond at SWMC landfill in February 2010 and was maintained in a condition with minimum exposure to oxygen. To avoid contamination, clean polyethylene containers were used to collect the samples. 44 All the parameters were determined within an hour and the remaining sample was stored in the refrigerator at 4°C until needed. The results are shown in Table 4.1. Table 4.1 Initial Physio-Chemical Characteristics of the Raw Leachate Sample (11th February 2010). Parameters Initial condition (before aeration) 21.6 Temperature (oC) Dark Brown Colour 110 Turbidity (NTU) 8.2 pH 1510 Total Alkalinity (as mg/L as CaCO3) 6.64 Electrical Conductivity (dS/m) 0.5 Dissolved Oxygen 600 Ammonia-nitrogen 8.4 Nitrate-nitrogen 0.2 Nitrite-nitrogen 13 Soluble reactive phosphorus Note:* All units in mg/L unless otherwise indicated. 4.1.4 Set-up of the aeration experiment Two tanks (A and B) of similar volume (223×10-6 m3) were filled with approximately 25,000 mL of leachate. Tank A was subjected only to natural aeration (without artificial aeration), while two aerators were fixed on Tank B to allow aeration. The setup of the experiment is shown in Figure 4.2. The flow rate of each aerator was approximately 250 L/hour. The laboratory was air conditioned at a constant temperature of 22±1oC. Approximately 100 mL of leachate from both tanks were sampled and analysed at intervals of approximately 48 hours for nine different parameters. In addition, DO and temperature were also monitored. The procedure was repeated until ten different readings were obtained on the 2nd, 4th, 6th, 8th, 10th, 12th, 14th, 16th, 18th and 20th days of aeration. The results are recorded in Table 5.1. 4.2 Column Experiments 4.2.1 Setup of the column experiments The experiment consisted of three columns (Column 1, 2 & 3) (Figure 4.3a), each having an overall height of 0.28 m and a cross sectional area of 0.0019 m2. These 45 Control Experiment Figure 4.2 Set up of the aeration experiment using glass-sided tanks and aerators showing Tank A (left) and Tank B (right). columns were designed to ensure uniform flow through the entire cross-section of the column. A polythene mesh was placed at the bottom of each column to prevent loss of the filter materials and clogging in the opening of the column. The columns were then filled with the filter material up to a height of approximately 0.2 m. They were lightly tapped to achieve some compaction and in order to allow uniform distribution of the filter material. The volume of media amounted to approximately 377×10-6 m3. The columns were mounted on a stand as shown in Figure 4.3b. Column 1 was a control while columns 2 and 3 functioned as the experiment and were designed to replicate one another. Once the columns were setup, 500 mL of de-ionized water was added to Column 1 under a constant head loading condition of 0.02 m. The same volume of leachate sample was added to columns 2 and 3. About 100 mL of sample was taken from each column, when the respective samples infiltrated through the column for the first time (1st run). The sample was then analyzed for nine different parameters as shown in Table 5.4. In the same manner, the remaining samples from each column were collected and recirculated ten times. This was done with the aim of obtaining another batch of samples (2nd run and 3rd run), each corresponding to different contact time between the sample and the filter materials. 46 a b Figure 4.3 Setup of the column experiment showing: a) different columns and b) constant head apparatus. The assumption was that with an increase in the number of filtrations or runs, greater contact times between the sample and filter materials resulted. However, only two batches of samples were taken, i.e., one at the end of the fifth run and another one at the end of the 10th run. The approximate contact times were estimated and presented in Table 5.3 in Chapter Five. The samples were finally analyzed and results recorded. The same procedure was repeated for BFS and sand. In summary, a matrix of 27 (3 - 1st run, 2nd run and 3rd run) × (3 columns) × (3 different filter materials) was obtained. All experiments were carried out at an approximate temperature of 22±1oC, inside an air conditioned laboratory. 4.2.2 Characterisation of leachate sample Although the leachate sample was collected from the same site as in the aerobic treatment, it was necessary to characterize the sample again before the experiment because samples were collected at different times of the year and hence, differ in their chemical characteristics. Thus, the initial analyses of the leachate sample and de-ionized water used for the column experiment are shown in Table 4.2. 4.2.3 Determination of physical and chemical properties of the filter materials Physical Properties of the Filter Materials Determination of pH, EC, bulk density, porosity percent and void ratio 47 The pH and EC of the filter materials were obtained by dissolving the respective materials in de-ionized water at a ratio of 1:5 (weight: volume). The solution was then agitated for 30 minutes at a speed of 300 OSC and the respective readings were recorded using pH and EC meter attached to HQ 40d DO probe. Table 4.2 Initial Physio-Chemical Characteristics of the Raw Leachate Sample and DeIonised Water Used in Column Experiments (18th May 2010). Parameters DeIonized Leachate Water Colourless Dark Colour Brown 0.3 59 Turbidity (NTU) 6.8 8.4 pH 30 4700 Total Alkalinity ( as mg/L as CaCO3) 15 10480 Electrical Conductivity (µS/cm) o NA NA** Temperature ( C) 9 2.2 Dissolved oxygen UMR* 975 Ammonia-Nitrogen UMR 0.2 Nitrite-Nitrogen UMR 10 Nitrate-Nitrogen 0.02 25 Soluble reactive phosphorus Note: All units in mg/L unless otherwise indicated. * Under Measuring Range. **Not Applicable. Bulk density, percent porosity and void ratio of each medium were obtained using the formulae below; Bulk Density (g/cm3) = Mass of known volume (g)/ Volume (cm3) Porosity (n) = Volume of voids (mL)/ Total Volume (mL) Void ratio (e) = Volume of voids (mL)/ Volume of Solids (mL) (Brady & Weil, 1996, p.114). Particle Size Distribution The sieve analysis undertaken in the laboratory involved a nested column of sieves with wire mesh (screen) of varying sizes from 5 mm to less than 0.063 mm. The sieves were placed in order of decreasing size, from top to bottom. A representative weighed sample (approximately 400 g) was poured into the top sieve, which has the largest mesh openings. Each lower sieve in the column has smaller openings than the one above and finally the sample is received at the base in a round pan. The column was then placed in a mechanical shaker. The shaker shakes the column for about 15 minutes to allow the 48 material whose diameter is smaller than the mesh opening pass through the sieves. After the aggregate reaches the final pan, the amount of material retained in each sieve was then weighed. The weight of the sample of each sieve was then divided by the total weight to give a percentage retained on each sieve (Equation 4.1). Equation 4.1 Calculation of filter material % retained on sieve (Eaton et al., 1995). where WSieve is the weight of material in the sieve and WTotal is the total weight of the material. The % retained was then subtracted from 100% to find the % passing (% finer by weight) through the sieve as shown in equation 4.2. Equation 4.2 Calculation of % finer by weight (Eaton et al., 1995). The values were plotted on a graph with percent passing on the Y axis and sieve size on the X axis (Figure 5.10 in Chapter Five). Determination of Hydraulic conductivity or permeability (Ksat-mm/hr) Based on the volume of influent, the surface area of the columns (0.00189 m 2), and using 1 mm =1 L/m2 and the time taken for the influent to infiltrate through the media, the hydraulic conductivity or permeability (Ksat-mm/hr) was calculated as shown in equation 4.3. Equation 4.3 Calculation of hydraulic conductivity of the filter material (Lucas, 2007, p.73). The summary of all the physical properties determined are presented in Table 5.2 in Chapter Five. 49 Chemical Properties Since the chemical properties of the filter materials were not determined in this study, the chemical properties determined by other researchers were referred as a source of information on the likely composition of the three filter materials used in this experiment. BFS is an industrial by-product resulting from steel-iron making processes (Kietlinska & Renman, 2005; Oguz, 2004). Due to high percentages of alumina and silica (Table 4.3), BFS is considered a good material for use as an economic adsorbent for large-scale use. The other reason is that it is easily available and affordable (Nehrenheim et al., 2008; Oguz, 2004) making it reasonable for use in the low cost treatment of waste water and leachates. Table 4.3 Typical Chemical Composition of BFS (amorphous-0.25-4 mm). Parameter % by weight. SiO2 35.5 CaO 35 Al2O3 9.6 FeO 0.3 MgO 13.7 MnO 0.4 S 1.4 V2O5 0.1 TiO2 1.7 Others 2.3 Source:Johansson (1999). Similar to BFS, the main components of the sand were SiO2 (69.3%), Al2O3 (13.4%), K2O (3.4%) and Fe2O3 (3.1%) (Kietlinska & Renman, 2005). In addition, it also contained traces of sodium (Na), magnesium (Mg) and calcium oxides. It is the finest of the three filter materials considered for this experiment. On the other hand, GAC is an entirely different filter material made of carbon. It is a good adsorbent medium due to its high surface area to volume ratio. One gram of a typical commercial activated carbon is reported to have a surface area equivalent to 50 1,000 m2 (Carbtrol Corporation, 1992). This high surface area permits the adsorptionof a large number of contaminant molecules. 4.3 Materials for Leachate Characterisation and Evaluation Three different data sets were obtained from different sources for the characterisation of leachate: Long term leachate monitoring data from the SWMC including leachate volume and rainfall, Climate data from the BOM, Australia website (www.bom.gov.au) retrieved November, 2010), Climate data of different regions in Bhutan obtained from Meteorology Section, Hydro-met Services Division, Department of Energy, MTI, Thimphu, Bhutan (1996-2009). In addition, secondary information about SWM systems in Bhutan were collected using previous research journals, documents and websites of different non government organisations, government departments and media. The data were analysed using various features of PASW Statistics 18 and Microsoft Word Excel 2007. T test, correlation and descriptive analysis were performed accordingly. 51 Chapter 5 Results This chapter presents the results obtained from two bench scale laboratory experiments. The results from the aerobic treatment of leachate are reported first followed by the column experiments using three different filter materials to ascertain their viability and suitability in leachate treatment. Both experiments of the research evaluated the possibility of treating leachate quality in a simple, affordable and effective way. 5.1 Aerobic Biological Treatment The results of the aerobic treatment of leachate are discussed in this section. The change in the characteristics of the leachate samples in the two treatment units (Tank A and Tank B) were monitored for a period of 20 days, using two glass sided tanks of 25 L capacity. Tank A was not aerated and functioned as a control for the experiment, whereas Tank B was aerated with two mechanical aerators. At an interval of every 48 hours, about 50-100 mL of leachate were obtained from the respective tanks (Tank A and Tank B) and analysed for a total of nine different physico-chemical characteristics (Appendix A-1). The overall outcomes of the study are presented in Table 5.1. As observed, the comparison of effluent quality between Tank A and B revealed that the aerobic treatment had a significant role in leachate treatment. A complete removal of ammonia-nitrogen (100%) and a reduction in the majority of turbidity (96%) were observed due to aeration in Tank B. Total alkalinity was also reduced by 40%. However, nitrogen compounds such as nitrite-nitrogen and nitrate-nitrogen rose due to aeration from an initial of 0.2 and 8.4 mg/L to 205 and 98 mg/L, respectively. These results were different to those in Tank A, which was not aerated and, which became anaerobic over the monitoring period. Ammonia-nitrogen and turbidity removal reduced to 29% and 46%, respectively. Total alkalinity increased from 1510 mg/L to 2520 mg/L as CaCO3. There was basically no change in nitrite-nitrogen and nitratenitrogen contents of the leachate in Tank A. 52 Table 5.1 The Change in Characteristics of Landfill Leachate in the two Tanks operated at 21ºC for 20 days. Tank A was the control while Tank B was aerated. Parameters Tank A Tank B Initial Effluent Removal % Effluent Removal% Colour pH Electrical Conductivity (dS/m) Turbidity (NTU) Total alkalinity (as mg/L as CaCO3) Dark Brown 8.2 6.64 Dark Brown 8.8 6.11 Increase Decrease Light Orange 8.8 5.78 Increase Decrease 110 1510 60 2520 46 Increase 5 910 96 40 Dissolved Oxygen Ammonia-Nitrogen Nitrite-Nitrogen Nitrate-Nitrogen 0.5 600 0.2 8.4 0.0 430 0.2 7.9 100 29 No Change Slight Decrease 4 UMR* 205 98 Increase 100 Increase Increase Soluble reactive phosphorus 13 13 No change 11 15 Temperature 21.6 2.5 No change 21.9 Slight Increase Note:* Under Measuring Range and all units in mg/L unless otherwise indicated. 53 One important observation was that a steady change in the leachate quality for all these parameters in both the tanks occurred roughly between the 4th and 6th day, indicating that even in a small volume tank a minimum of six-day detention time is required to significantly reduce the concentration of a number of the contaminants. Further details of the variations for individual parameters as a function of aeration time are shown in Figures 5.1, 5.2, 5.3, 5.4, 5.5, 5.7, 5.8 and 5.9. 5.1.1 Change in the nitrogen compounds One of the significant changes in the quality of a leachate is demonstrated by the reduction in ammonia content. Figure 5.1 demonstrates that the concentration of ammonia-nitrogen in both the tanks varied significantly over a period of 20 days. For the first four days, there was an abrupt increase in ammonia-nitrogen from an initial concentration of 600 mg/L to 1180 mg/L in Tank A and 1385 mg/L in Tank B. Figure 5.1 The changing pattern of ammonia concentration in Tank A and Tank B over a period of 20 days. However, with an increasing aeration time, the ammonia-nitrogen concentration began to decline rapidly and by the 16th day, a complete removal of ammonia-nitrogen was achieved (0 mg/L) in Tank B. Azevedo (1993) reported the same findings. In Tank A, there was no further decrease and the concentration stabilised at 430 mg/L. This corresponds to an overall ammonia removal of approximately 29% and 100% for Tank A and Tank B, respectively. 54 The changes could be attributed to the conversion of ammonia to other forms of nitrogenous compounds, particularly nitrate in the presence of oxygen (Berge et al., 2006; Cook & Foree, 1974). The two small aerators together had a flow rate of 500 L/hour (equivalent to approximately 8 L/Min); therefore aeration is the principal factor behind the steady concentration (2-4 mg/L) of DO maintained in Tank B. In Tank A, the DO was reduced to 0 mg/L within six days. The difference indicates that there was no available oxygen for the oxidation of ammonia in Tank A. If nitrification is considered as the main mechanism for ammonia reduction, a substantial decrease in ammonia would simultaneously result in an increase in nitrite and nitrate contents of the leachate. This is reflected in Figures 5.2 and 5.3, which demonstrate the changes in nitrite and nitrate nitrogen concentration in the respective tanks. Figure 5.2 The changing pattern of nitrite concentration in Tank A and Tank B over a period of 20 days. In Tank B, there was a gradual increase in both nitrite-nitrogen and nitrate-nitrogen, from an initial concentration of 0.2 mg/L and 8.4 mg/L, respectively to 205 mg/L and 98 mg/L particularly from the 8th day onwards. Interestingly, this time period coincided with the time at which ammonia-nitrogen (6-8 days) began to decrease, suggesting that 55 the decrease in ammonia and the increase in nitrite and nitrate were associated with the development of nitrification. Figure 5.3 The changing pattern of nitrate concentration in Tank A and Tank B over a period of 20 days. However, the observation is not the same in Tank A. The slight decrease in ammonianitrogen (29%) did not influence the nitrite and nitrate nitrogen concentrations. Instead, it was observed that their concentrations remained constant at 0.2 and 7 mg/L, respectively, as the only oxygen to enter the tank was through entrainment from the atmosphere above. In this case there was not sufficient oxygen in the leachate for nitrification to occur. Earlier studies reported similar observations stating that below 2 mg/L, the process of nitrification is inhibited resulting in a nitrite build up. Further, at a lower DO of 0.5 mg/L, both nitrite accumulation and ammonia consumption decreased (Bickes & Van Oostron; cited in Shao et al., 2008). 5.1.2 Turbidity Turbidity is caused by suspended and colloidal matter such as clay, slit, finely divided organic and inorganic matter, plankton and other microscopic organisms. Therefore, a decrease in turbidity of the leachate may indicate decrease in these contents. In this experiment, there was a substantial decrease in turbidity. This was most notable in Tank B (Figure 5.4). 56 Figure 5.4 The changing pattern of turbidity in Tank A and Tank B over a period of 20 days. Firstly, in both tanks the turbidity of the leachate declined rapidly within the first four days. In Tank A, turbidity decreased from an initial of 110 NTU to 50 NTU while in Tank B with aeration it was reduced to 25 NTU. While further increase in detention time did not have an effect on turbidity in Tank A, the turbidity of the leachate continued to decrease in Tank B. As a result, at the end of the 20th day, approximately 96% of turbidity was removed in Tank B, which is much higher than 46% removal in Tank A. The results demonstrate that aeration has a significant effect on turbidity and can improve the leachate quality. It should also be noted that in Tank B, a small portion of the solids may have been removed by the filtering material in the aerators. Turbidity removal in both the tanks could also be associated with the reduction in ammonia earlier, given that a large majority of organics initially present in the leachate can be very well degraded under both aerobic and anaerobic conditions (Gourdon et al., 1989). However, the level of organic removal is much higher in an aerobic condition (Tank B) because a large part of the OM not removed by the anaerobic treatment was readily biodegradable in aerobic conditions. A comparision of figures 5.1 and 5.4 show this occurrence. 57 5.1.3 Electrical conductivity (EC) The EC of the leachate in Tank A decreased from an initial of 6.64 dS/m to 6.11 dS/m while in Tank B the final EC was lower at 5.78 dS/m (Figure 5.5). It is therefore possible the ionic solutes might have been removed because they are generally suspended in the solution along with other organic materials. Figure 5.5 The changing pattern of EC in Tank A and Tank B over a period of 20 days. 5.1.3 Colour and odour A visual assessment of the difference in leachate quality from each tank at the end of the test period is shown in Figure 5.6. The improved clarity of the aerated sample (right) is shown. Similarly, sensory records showed an improvement in odour of the leachate after aeration. 58 Figure 5.6 The difference in colour of the leachate samples from Tank A (left) and Tank B (right). 5.1.4 pH and total alkalinity Although, the fluctuations in pH were much less in Tank A as with the case of total alkalinity, both the parameters fluctuated to a greater extent in Tank B. That is, pH became increasingly alkaline over the period (8.2-8.8) in both the tanks; however, the fluctuation was greater in Tank B (Figure 5.7). Figure 5.7 The changing pattern of pH in Tank A and Tank B over a period of 20 days. 59 Similarly, the total alkalinity of the leachate changed over the monitoring period as shown in Figure 5.8. For the first six days, there was a two-fold increase in total alkalinity values in both the tanks (1510 to 2860 in Tank A and to 2670 mg/L as CaCO3 in Tank B). However beyond the sixth day, the changes in the respective tanks differed. That is in Tank A, no further reduction was observed whereas in Tank B, a 40% reduction was observed. Figure 5.8 The changing pattern of alkalinity in Tank A and Tank B over a period of 20 days. 5.1.5 Phosphorus Phosphorus in landfill leachate includes organic and inorganic phosphorus. This nutrient can cause detrimental eutrophication in aquatic environments (Wang et al., 2010). In leachates, phosphorus can be measured as SRP, which is a chemically active dissolved form of phosphorus readily available for biological uptake. A particular concern with this pollutant is that it is readily available to algae and under certain conditions can stimulate excess algae growth leading to subsequent depletion of DO. Apart from leachates, SRP is found in wastewater treatment plants, feedlot runoff, and failing septic systems. To examine the effect of aerobic treatment on this pollutant, the changing pattern of Pconcentrations in the leachate in each tank was examined as shown in Figure 5.9. 60 Figure 5.9 The changing pattern of phosphorus in Tank A and Tank B over a period of 20 days. The leachate sample initially contained approximately 13 mg/L of phosphorus. Although a slight increase (11.8-16.6 mg/L in Tank A and from 11 to 17.6 mg/L in Tank B) was observed between day 6 and day 10 of aeration, the final P-concentration in Tank A remained the same. In Tank B, the P-concentration was reduced to 11 mg/L, approximately equivalent to a 15% reduction. However, the final change was minimal, demonstrating that both aerobic and anaerobic conditions do not have great influence on the P-concentration in the leachate. 5.2 Column Experiments The laboratory method associated with these column experiments has previously been described. Similar to the first experiment, nine different parameters of a typical leachate from the SWMC were considered for the investigation. The parameters analysed were colour, turbidity, pH, electrical conductivity, total alkalinity, ammonia-nitrogen, nitritenitrogen, nitrate-nitrogen and SRP(Appendix A-2) 61 Operating conditions Several factors considered in operating the column experiment were: the mass, bulk density and particle size distribution of the filter materials, the contact time and hydraulic conductivity (permeability), the volume of sample absorbed due to the differences in porosity. Mass, Bulk Density and Particle Size Distribution The purpose of determining mass and more importantly the bulk density of the filter material was to investigate if specific filter materials were more effective in the treatment of leachate than others. The mass of filter materials required in filling up a column volume of 377×10-6 m3 was determined as 0.09 kg of GAC, 0.45 kg of BFS and 0.485 kg of sand (Table 5.3). Bulk densities further illustrate their mass per volume (kg/m3) distribution, which was determined by the method discussed in Section 4.2.3. The bulk density values for GAC, BFS and sand are determined as 300 kg/m3, 1500 kg/m3 and 1600 kg/m3, respectively (Table 5.2). With 300 kg/m3, some particles of the GAC floated in the leachate sample. The particle size distribution graph (Figure 5.10) was obtained from Appendix A-3 and used to estimate the effective size (D10) of each filter material. The D10 values are also presented in Table 5.2. 62 Table 5.2 Physical Properties of Three Different Filter Materials: GAC, BFS and sand. Parameter Type of media GAC BFS Sand Black Grey Pale Yellow Colour 9.4 10.9 6.5 pH 526 930 10.6 Electrical Conductivity (µS/cm) 2.8 0.5 0.25-0.125 Effective Particle size (mm) (D10) 68 40 36 Porosity (%) 3 300 1500 1600 Bulk Density (kg/m ) 2.1 0.7 0.6 Void Ratio 192 29 32 Hydraulic Conductivity (m/day) Note:* Effective size D10 was calculated from Figure 5.10. Table 5.3 Materials GAC BFS Sand Mass of materials (kg) 1st Run 0.090 0.450 0.485 3 14 12 Operating Parameters for the Column Experiments. Contact time (mins) Volume of sample permeated (mL) nd rd 2 3 1st 2nd 3rd Run Run Run Run Run 15 70 60 30 140 120 500 500 500 350 370 350 260 290 260 63 Figure 5.10 Plot of % of media finer by weight as a function of particle size (mm). Contact Time and Hydraulic Conductivity A total of three runs was considered for the whole column experiment - 1st run, 2nd run and 3rd run. Basically, each run was a flow of leachate sample at a rate determined by the filter materials (volume = 377×10-6 m3) under a constant head loading (0.02 m) condition. However, the 2nd and 3rd runs were obtained by recirculating the leachate samples for five and ten times, respectively and hence were comprised of numerous single runs. The important aspect of each run is that it is basically determined by the filter material’s saturated hydraulic conductivity (Ksat). The purpose of determining Ksat was to gain insight into the rate at which the leachate samples flowed through the filter medium in the columns. The Ksat values for the three different filter materials are also presented earlier in Table 5.2. GAC exhibits a comparatively higher hydraulic conductivity (192 m/day) than BFS (29 m/day) and sand (32 m/day). The other important point is that hydraulic conductivity plays an important role in determining the contact time period between the material and the influent. That is, a material with lower hydraulic conductivity induces higher contact time and vice versa. 64 The actual contact time in this experiment was estimated as the total time taken by the 500 mL of sample to permeate through the respective filter material columns under a constant head loading condition. Therefore, the contact time determined from 1st run was multiplied by five and ten times, respectively to obtain a total of three different contact times in an increasing order. GAC provided the least contact time period in all the runs (1st, 2nd and 3rd) with three, 15 and 30 mins, respectively, whereas BFS materials ensured a significantly higher contact time of 14, 70 and 140 mins, respectively. Finally, sand also had similar contact time period to that of BFS with the first contact time period calculated as 12 mins and the remaining as 60 mins and 120 mins, respectively (refer Table 5.3 before). Volume of Sample Absorbed Another important operating parameter is the ability of filter materials to absorb a certain volume of a sample. This volume was calculated by subtracting the effluent sample volume from the influent. In the first run, GAC and sand columns demonstrated that the filter materials roughly absorbed 100 mL of sample whereas BFS could absorb only 80 mL. In the 2nd run, the absorption reduced to 40 mL for GAC and sand and 30 mL for BFS. The difference in the ability of the filter material to absorb sample is apparently due to differential % porosities of the filter materials (GAC (68%), BFS (40%), and sand (36%)) (Table 5.2 before). Unlike the 1st and 2nd runs, the 3rd run did not absorb the samples, indicating that all the filter materials were already saturated and thus no absorption occurred. In addition, about 50 mL of the effluent sample from each run was collected for analysis. The consequence of the absorption phenomenon is that it varied the volume of samples permeated in the 2nd and 3rd runs. For example, although 500 mL of sample were permeated in all the filter columns in the 1st run, the volumes of samples permeated in the 2nd and 3rd runs differed from one column to the other. For example, in the 2nd run, only 350 mL of sample was permeated in the GAC and sand columns and 370 mL in the BFS columns. Similarly, there was a substantial decrease in the volume of sample permeated in the 3rd run with approximately 260 mL for GAC and sand and 290 mL for BFS. 65 Column Experiment Results The outcomes of the leachate treatment from the filter materials are presented in Table 5.4. The table presents both range and mean values of the nine parameters examined to determine the overall impact of the filter materials on leachate treatment. 5.2.1 Solube reactive phosphorus removal The percentage (%) removal results in Table 5.4 show the filter materials have most significant impact on leachate sample with respect to P-removal. The final effluent Pconcentrations demonstrated a significant decrease with an estimated P-removal of approximately 68%, 92% and 40% for GAC, BFS and sand, respectively. Earlier, Johansson (1999) investigated BFS in a laboratory scale column experiment. The results indicated that this material had a high P-sorption potential to remove more than 90% of phosphorus from a P-solution at concentration 10 mg/L. Figure 5.11 further illustrates the pattern of decrease in P-concentrations as a function of three different contact times. Figure 5.11 The changing level of phosphorus in the leachate samples following different runs throughout the experiment. 66 Parameter Colour pH Electrical Conductivity (dS/m) Turbidity (NTU) Alkalinity (as mg/L as CaCO3) Ammonia-Nitrogen Nitrite-Nitrogen Nitrate-Nitrogen Soluble Reactive Phosphorus Table 5.4 Mean Results Obtained from Column Experiments using three Different Filter Materials. GAC BFS Sand % Effluent Effluent Effluent Effluent % Effluent Effluent Removal (range) Influent (range) (mean) (mean) Removal (range) (mean) % Removal Dark 8.4 Brown NA 8.7-8.7 Black 8.7 NA NA NA 9.4-9.6 Light 9.5 Brown NA NA NA 8.7-8.8 Dark 8.8 Brown NA NA 10.48 10.1210.18 10.15 NA 9.83-9.9 9.865 NA 9.97-10.06 10.015 NA 59 202-209 205 NA 45-46 46 22 42-43 43 25 4700 4420-4780 4600 2 1450-1780 1615 66 4280-4440 4360 7 975 0.2 10 1060-1080 0.3-0.3 4.7-8.3 1070 0.3 7 NA NA 30 1010-1265 0.1-0.1 20-22 1138 0.1 21 NA NA NA 1060-1260 0.2-0.2 12.8-12.8 1160 0.2 13 NA NA NA 25 7-9 8 68 2-2 2 92 14-16 15 40 Note: All the units in mg/L unless otherwise indicated. NA indicates Not Applicable for % removal estimation. 67 For all the materials, the maximum reduction in phosphorus (app. 64% and 76% and 28%, respectively) occurred during the 1st run (initial phosphorus~25 mg/L) corresponding to a contact time period of 3, 12 and 14 mins, respectively. Further reductions in P-concentration were observed in the subsequent runs essentially due to the fivefold increase in contact time estimated as 15, 60 and 70 mins, respectively. Beyond the 2nd run, there was no major decrease in P-concentration possibly indicating the saturation of the materials. This trend signified that a longer contact time resulted in a greater P-removal. However, beyond the 2nd run, the 3rd run did not lead to greater P-removal, which is an indication that an additional increase in contact time would not result in adsorption. The results illustrate that although the same volume (377×10-6 m3) of filter material and the same volume of leachate (500 mL) were considered, each filter material has a different response towards phosphorus, thus resulting in various P-removal efficiencies at different contact times. P-sorption capacity The objective of this part of the analysis was to evaluate sorption and removal of phosphorus from model solutions by various filter materials in batch experiments. This was carried out by developing P-sorption isotherm (curves) and could possibly verify the assumption that P-sorption by filter materials is the main mechanism behind Premoval. For this purpose, batch tests results for P-sorption were used. The batch test was conducted by Lanfax Laboratories according to the Five Point P-sorption Curve method used for determining P-sorption. The details of the method are as outlined in Patterson & Jones (2001): Mass of sample: 4.0g Phosphorus concentration: 25, 50, 75, 100 and 150 mg P/L Sample/Solution ratio: 1:10 Equilibrating solution: 0.01M CaCl2 Tumbling period: 17h at 25oC. The resulting P-sorption isotherms or curves are shown in Figure 5.12. 68 Figure 5.12 P-sorption isotherms for three different filter materials developed using five different P-solutions in the batch scale experiment (Lanfax Laboratories). According to the P-sorption curves, P-sorption increases proportionally with the increase in initial phosphorus concentration. Nevertheless, P-sorption capacity from 25 mg/L phosphorus solution was selected to resemble the P-concentration of the leachate sample used in this experiment. When fed with 25 mg/L of phosphorus, the three filter materials-GAC, BFS and sand- had a capacity to adsorb 249, 257 and 27 mg of phosphorus for every one kilogram of the material, respectively. 5.2.2 Turbidity and colour Apart from a significant P-removal, there was a considerable decrease in the turbidity of the effluent leachates treated with BFS (22%) and sand (25%) as shown in Table 5.4. This might be an indication that the filter materials removed a considerable amount of suspended and colloidal matter possibly containing clay, slit, and finely divided organic and inorganic matters during the filtration. Aziz et al. (2007) suggested that high levels of OM (measured as COD) are associated with turbidity and suspended solids. However the drastic increase in turbidity from 59 NTU to 205 NTU in the effluents from the GAC column was not anticipated (Figure 5.13). 69 Figure 5.13 The changing level of turbidity in the leachate samples following different runs throughout the experiment. In addition to turbidity reduction, there was an improvement in colour of the leachate before and after the treatment, particularly effluent from the BFS and sand columns. Hence, the overall improvement in the turbidity of the leachate is confirmed by the changes in the colour of the leachate effluents (Figure 5.14 b, c) based on a visual assessment. GAC (Control) GAC (Experiment) a) 70 BFS (Control) BFS (Experiment) b) Sand (Control) Sand (Experiment) c) Figure 5.14 The difference in the colour of the leachate samples for three different runs along with their respective controls. a) GAC, b) BFS and c) sand. 5.2.3 Electrical conductivity Further, adsorption of other forms of dissolved solids (particularly inorganic solutes) present in the leachate is evident from the fact that all the filter materials were able to reduce the EC of the leachate sample. This is shown in Figure 5.15. The EC values decreased from an initial 10.480 dS/m to a final of 10.15 dS/m, 9.865 dS/m and 10.015 dS/m for GAC, BFS and sand, respectively. Although, all the materials resulted in a reduction of EC, effluent samples from BFS demonstrated extreme EC variations at different runs. For example, there was a sudden initial increase in EC from 10.48 to a very high 10.795 dS/m in the 1st run. The values, however, decreased eventually to 10.155 dS/m and finally to 9.865 dS/m in the 2nd and 3rd runs, respectively. The results reflect that BFS might have initially released certain dissolved solids into the effluent and that with repeated circulations it showed an ability to re-adsorb them. However, this did not happen to the effluent from GAC and sand. 71 Rather a substantial decrease was observed with each increasing run as shown in Figure 5.15. Figure 5.15 5.2.4 The changing level of EC in the leachate samples following different runs throughout the experiment. Total alkalinity and pH The total alkalinity removal ability for different filter materials is shown in Figure 5.16. Over all, a significant reduction in total alkalinity (66%) was observed in the effluent sample treated with BFS. The reduction was, however, less in the case of GAC and sand with 2% and 7%, respectively. In the BFS columns, a significant reduction in P-removal occurred at the 2nd run. Hence, the final total alkalinity value of 1615 mg/L as CaCO3 corresponded to a 66% decrease. The only possible explanation could be that BFS materials might have released acids that can react with bicarbonates present in the leachate and possibly converted them reducing the total alkalinity. Therefore, low total alkalinity values indicated that the leachate no longer had ability to buffer against any pH changes in the environment, thus becoming more prone to fluctuations in its quality, especially with respect to its acidity and alkalinity. 72 Figure 5.16 The changing level of alkalinity in the leachate samples following different runs throughout the experiment. On the other hand, the changing pattern of the effluents treated with GAC and sand are almost consistent and consequently the final values are only slightly lower than the initial - 4600 and 4360 mg/L of CaCO3, respectively. Figure 5.17 demonstrated that with each run, the effluent leachate samples became more alkaline. This change was common to all the filter materials although it was significantly high in the case of the effluent treated with BFS. pH values for BFS effluent increased from 8.4 to 9.5 and to a little over 8.7 and 8.8 for GAC and sand respectively. 73 Figure 5.17 The change in pH values of the leachate samples following different runs throughout the experiment. 5.2.5 Ammonia-Nitrogen, Nitrite-Nitrogen and Nitrate-Nitrogen Figure 5.18 demonstrates that ammonia concentrations in the leachate in all the three columns reached a maximum during the experimental period. For example, ammonianitrogen concentration increased from an initial 975 mg/L to 1178 mg/L in the 1 st run for GAC effluent, to 1108 mg/L for BFS and to 1243 mg/L for sand both in the 2nd run. Figure 5.18 The changing level of ammonia in the leachate samples following different runs throughout the experiment. 74 In terms of nitrite-nitrogen, effluent values for nitrite from all three columns were similar and fluctuated only by ±0.1 as compared to the initial concentration (0.2 mg/L). Thus, it can be assumed that there was no change in nitrite; instead, it remained remarkably constant throughout the experiment. The effluent samples treated with BFS showed a significant two fold increase in nitratenitrogen from an initial of 10 mg/L to 27 mg/L in the 2nd run and then to 21 mg/L in the 3rd run (Figure 5.19). This was not the case with the effluents from GAC and sand columns because the nitrate-nitrogen concentration only fluctuated by negligible amounts and as such there were no significant change throughout the entire experimental period. Therefore, it may be concluded that GAC and sand neither contributed to an increase nor to its removal from the effluent. Figure 5.19 The changing level of nitrate in the leachate samples following different runs throughout the experiment. One relation from the Figures 5.18 and 5.19 suggests that when nitrate-nitrogen concentrations for BFS effluents reached maximum, the ammonia-nitrogen concentration was comparatively low. Conversely, GAC and sand with no or little change in nitrate concentration showed more fluctuations in ammonia-nitrogen. 75 Chapter 6 Discussion The chapter is divided into two sections. Both sections discuss the overall outcomes of the two bench scale laboratory experiments and their relevance and suitability as a low cost passive leachate treatment option in Bhutan. The changes in the quality of the leachate from both experiments are discussed in detail. The application and limitations of this treatment study including concerns with respect to their application as an onsite leachate treatment plant in Bhutan are outlined. 6.1 Aerobic Biological Treatment This experiment followed earlier bench and full scale treat-ability studies, which showed that raw leachate could be effectively treated by aerobic biological processes and that complete nitrification of ammonia could be achieved under suitable conditions (Boyle & Ham, 1974; Cook & Foree, 1974; Knox, 1983 & 1985; Robinson & Grantham, 1988; Robinson & Maris, 1983) The bench scale treatment process was designed to further investigate the treatment of leachate in onsite landfill facilities and to particularly resemble the facultative treatment lagoons. The experiment was carried out in two glass-sided tanks over a period of 20 days and involved 25 L of medium strength leachate obtained from the SWMC landfill. A mean temperature of approximately 21oC was maintained over the period of test to simulate conditions at onsite leachate treatment plants. There was no pH manipulation or nutrient addition. In Tank B, the two aerators together have a capacity to aerate 500 L of leachate per hour that enables a minimum oxygen concentration of 2 mg/L – the required limit for most aerobic treatment systems. Tank A was not aerated and thus served as a control for the experiment. Further the experiment demonstrated that even in a small volume tank a minimum of six-day detention time is required to significantly reduce the concentration of a number of the contaminants. This could be the minimum period of time required to facilitate biodegradation in the leachate in both tanks. The four to six day time period has a close similarity to those reported by Yahmed et al. (2009) and Robinson & Maris (1983). 76 Although certain operating conditions differed from this experiment, the aerobic treatments of leachate were all based on smaller scale pilot experiments in the laboratory. Yahmed et al. (2009) demonstrated that a significant OM reduction (indicated by TOC) was achieved from an undiluted young leachate in bioreactors at a detention time of 108 hours (~ four and one half days). Similarly, a well clarified effluent and a high removal of BOD (>98%) and COD (>92%) was obtained from a medium strength leachate at a slightly higher detention time of 10 days (Robinson & Maris, 1983). 6.1.1 Ammonia-nitrogen removal Initially, during the first four days, an abrupt increase in ammonia was observed in both the tanks. Azevedo (1993; cited in Shiskowski & Mavinic, 1997) described this phenomenon as a sort of ammonia “spike” believed to be occurring in both anoxic and aerobic reactors when ammonia load is initially high. This could be attributed to the rapid biodegradation of organic nitrogenous compounds in the leachate. Although, the biological degradation is lesser in Tank A due to anaerobic conditions, ammonianitrogen accumulation still occurred due to its stability under anaerobic conditions. In addition, anaerobic conditions do not have ammonia elimination process (Vigneron et al., 2007; cited in Shou-liang et al., 2008). However, a 100% ammonia-nitrogen removal in Tank B and 29% in Tank A at the end of the experimental period suggested that ammonia removal was achievable, especially in Tank B. This could be attributed to complete nitrification of ammonia brought about by an intensive aeration at a higher pH of 8.2-8.8 optimum for nitrification. In Tank A, the slight reduction in ammonia content could be due to the volatisation of ammonia to the atmosphere (Robinson & Maris, 1983) or through the assimilation by anaerobic bacteria, which utilises ammonia for cellular growth over the period of the test (Kettunen et al., 1996; cited in Shou-liang et al., 2008). The mechanism for the conversion of ammonia to nitrate takes place by the presence of bacteria known as “nitrifiers”, which are strict “aerobes,” meaning they must have free dissolved oxygen to perform their work. Therefore, nitrification occurs only under aerobic conditions at DO concentrations of 1.0 mg/L or more. Nitrosomonas first 77 convert ammonia and ammonium to nitrite and then Nitrobacter finish the conversion of nitrite to nitrate. The reactions are generally coupled and proceed rapidly to the nitrate form; therefore, nitrite concentrations at any given time are usually low. At DO concentrations less than 0.5 mg/L, the growth rate is minimal; hence nitrification is inhibited in anaerobic conditions. The 100% ammonia-nitrogen removal in Tank B could also mean the main constituent of concern in the raw leachate, which can cause a range of serious problems such as eutrophication, increase in BOD and stimulation of algal growth in water systems, was successfully treated (Berge et al., 2006; Boyle & Ham, 1974; Cook & Foree, 1974; Knox, 1985; Pivato & Gaspari, 2006; Wang et al., 2006; Wang et al., 2008). The treated effluent is free of ammonia (0 mg/L) and therefore suitable to be discharged into the environment according to this parameter. For instance, in Australia, the AWQGFMW (1992) allows 0.5 mg/L of ammonia in the fresh and sea water systems. In case the effluent does not meet this discharge limit, it will still be suitable for discharging into the municipal waste water treatment or sewerage systems. An important observation in Tank B is that the pH and total alkalinity of the leachate sample fluctuated more significantly than in Tank A. The changes might be due to the process of nitrification that can produce acid. Acid formation might have caused fluctuations in pH, in most cases lowering its value by a few units. However, it did not affect the nitrification process because pH values (8.2-8.8) still fell in the optimum range for the nitrifiers given that most treatment plants are able to effectively nitrify above a pH of 6.5. The assumption compared favourably with Tank A where fewer changes in pH occurred due to the absence of nitrification. In addition, the nitrification reaction (that is, the conversion of ammonia to nitrate) is reported to consume 7.1 mg/L of total alkalinity as CaCO3 for each mg/L of ammonia nitrogen oxidized. This occurrence might have resulted in a reduction of total alkalinity in Tank B. Further observations are that reduction in ammonia-nitrogen, total alkalinity and increase in nitrite-nitrogen and nitrate-nitrogen all occurred simultaneously between the 6th and 10th days of aeration. So, it may be correct to assume that about 40% of total alkalinity is consumed for 100% ammonia-nitrogen reduction. However, the reduction 78 did not affect the buffering ability of the leachate because 900 mg/L as CaCO3 is above the minimum total alkalinity requirement (50-100 mg/L as CaCO3) for a treatment tank. Conversely, in Tank A, where nitrification was absent, both ammonia-nitrogen and total alkalinity reductions were insignificant or minimal. Nitrite and nitrate nitrogen concentrations also remained consistent. 6.1.2 Turbidity and electrical conductivity Apart from the nitrogenous compounds, leachates contain significant amounts of organic and inorganic solids indicated variously by colour, turbidity and EC. The results of the change in these parameters in the presence and absence of aeration show a thorough understanding of how aerobic treatment can bring about significant improvement in the quality of the final effluent. The first is a decrease in turbidity that partly reflects the OM content of the leachate. Therefore, although the BOD, COD and TOC indicators were not monitored in this study to model biodegradation of leachate, it is noticeable from the turbidity that substantial reduction in OM occurred from the 4th day onwards in both the tanks. By comparison, the OM was almost completely removed (96% reduction in turbidity) in Tank B and to a much lesser extent in Tank A (46%). The results are consistent with the findings of Boyle & Ham (1974), Cook & Foree (1974) and Robinson & Marris (1983), where a substantial decrease in the OM was observed at the same retention time for leachates containing high amounts of OM. That is, when leachates with high BOD/COD values (1550/2700 mg/L, 3000/5000 mg/L and 7100/15800 mg/L) were aerobically treated at a bench scale, it resulted in more than 80% BOD and COD removal at a five day retention time. The second important parameter of the leachate is the EC. It reflects the total concentration of ionic solutes present in a solution (Jun et al., 2009). The slight decrease in EC in both the tanks can be associated with the removal of ionic solutes. However, as compared to OM, ionic solute removal is less. This is because the leachate sample is likely to contain relatively high amounts of elements such as Na, K, Ca, Mg and so on. Since none of this can be lost from the leachate simply by aeration, there was only a slight influence on EC. 79 6.1.3 Colour and odour The combined effect of reduction in turbidity and EC improved the clarity (colour) of the aerated sample, particularly in Tank B where the colour of the leachate changed from dark brown to clear light orange after the aerobic treatment (Figure 5.6 ). In addition, aeration had a postivive impact on removing the foul odour of the leachate possibly due to the degradation in OM in Tank B. Conversely, in Tank A, foul odour was developed over the period of time. 6.1.4 Phosphorus The aerobic treatment experiment exhibited that aeration didnot have influence on the phosphorus concentration in the leachate. With the exception of Cook & Foree (1974), who asserted that aeration resulted in a removal of Total-phosphorus and soluble reactive phosphorus from leachates in 10 days, it is possible there is no literature of the positive effect of aerobic treatment for phosphorus. Instead, P-removal was mostly based on three factors such as precipitation; adsorption or the assimilation by certain micro-organisms present in both anaerobic and aerobic conditions. The same assumption might possibly explain the difference in fluctuations of P-concentrations in the respective tanks. Although all three factors might have contributed to the fluctuations in P-concentration in both the tanks, the slight reduction in phosphorus in Tank B must be due to the greater possibility of removing phosphorus by micro-organism assimilation in aerobic conditions (Sedlak, 1991). According to this author, all biological phosphorus removal systems utilise the following two-step process description: “Certain micro-organisms when subjected to anaerobic condition assimilate and store fermentation products produced by other facultative bacteria. These micro-organisms derive energy for this assimilation from stored polyphosphates, which are hydrolysed to release energy. The resulting phosphorus is released to the mixed liquor. These same micro-organisms, when subsequently exposed to aerobic conditions consume both phosphorus and Oxygen to metabolize the previously stored substrate for energy production and cell synthesis” (Sedlak, 1991, p.167). 80 6.1.5 Temperature Although aerobic treatments are sensitive to temperature and changes in temperature are likely to bring certain changes in the treatment efficiency, at normal temperatures, it may not pose much effect on treatment efficiency. Earlier, Robinson & Maris (1983) studied the effect of cold temperature (10oC) on aerobic treatment and at 20-25oC by Cook & Foree (1974). The experiments confirmed that an efficient removal of OM occurred at 10 days in both conditions. This indicated that within reasonable temperature changes, the effect should not be as significant. Hence, unless the temperature is completely extreme, treatment of leachate is still feasible. In the present study, temperature remained almost constant throughout the experimental period with values ranging between 21.3-21.9oC in Tank A and 21.4-22oC in Tank B. Both resembled onsite temperatures and are basically suitable for OM degradation and nitrification. In fact the slightly higher temperature in Tank B might be due to the rapid activity of microorganisms. However, the heat produced was possibly offset by the cooling effect of aeration. With regard to the effect of temperature on nitrification, nitrification ceases only at temperatures higher than or equivalent to 40oC and below 10oC and thus, between this range, temperature does not have much effect on the treatment process. Berge et al. (2007) reported that ammonia removal by the process of nitrification in a bioreactor landfill leachate can readily occur over a range of temperatures (22oC, 35oC and 45oC.). However, it was observed that ammonia removal was most efficient at 35oC and slower at 22oC and 45oC. 6.1.6 Retention time The most important factor to consider in an aerobic treatment is the retention time. Graphical illustrations of various pollutant parameters as a function of time in Section 5.1 showed the influence of retention time on the treatment efficiency in both tanks. Firstly, a careful assessment of the change in leachate characteristics as a function of time reveals that both aerobic and anaerobic treatment requires a minimum six day retention time to establish a steady state or stabilize. The first four to six days did not 81 bring about any steady change in the leachate quality. Instead, this period was characterized by abrupt changes that were not consistent with the remaining experimental period. As an illustration, the rapid increase in ammonia-nitrogen, total alkalinity and P and the decline in turbidity in both the tanks, occurred between the 4th and 6th day (see Figures 5.1, 5.8, 5.9 and 5.4, respectively). Hence, the first four to six days is considered the minimum retention time required in facilitating biodegradation of leachate, irrespective of whether the condition is aerobic or not. The fact that the above changes were common in both tanks in the initial period implies that leachates also have a natural tendency to undergo stabilization in aerobic as well as anaerobic conditions. Rapid biodegradation of OM might have led to the rapid increase in ammonia and decrease in turbidity during that period. In an anaerobic condition (Tank A), this might have been possible due to the presence of dissolved oxygen initially present in the leachate. Consumption of oxygen was evident from the fact that dissolved oxygen was completely reduced to 0 mg/L within four days in Tank A. Secondly, it is evident that longer retention time beyond six days brought significant changes in the quality of leachate. Around the 16th day, a final steady change was observed with the exception of P. At this time, in Tank B, the leachate quality improved significantly with complete removal of ammonia and turbidity. Total alkalinity and EC were reduced by considerable units, while nitrite and nitrate nitrogen attained maximum concentrations and remained remarkably constant thereafter. In Tank A, a considerable decrease in the concentrations of the pollutants occurred. Beyond the 16th day, there was no significant change in the leachate quality in both the tanks, hence the 16 days is considered an approximate retention time for both aerobic and anaerobic treatment in this study. A 16 day retention time compared favourably with other bench scale studies that considered a 10 day retention time more effective than five day at normal operating temperatures of 20-25oC. In addition, BOD and COD removal efficiency of 80-90% at a 10 day retention time declined to about 40-50% when the retention time is lowered by five days (Boyle & Ham, 1974; Cook & Foree, 1974; Robinson & Maris, 1983). However, a longer retention time would be more appropriate and desirable when large 82 scale leachate treatment is involved. In onsite treatment systems, a higher retention time of 32 days (Frascari et al., 2004) and 56 days (Mehmood et al., 2008) were required to achieve similar removal efficiencies. Therefore, the retention time is an important controlling factor for both aerobic and anaerobic treatment of leachates and should ideally be 10 days and not less than six days. 6.1.7 Application The suitability of aerobic treatment of leachate was suggested by Boyle & Ham (1974) and Cook & Foree (1974). The concept has, however, mostly been used as a pretreatment step prior to a biological treatment in a municipal facility (Frascari et al., 2004). The results obtained from this study along with Matthews et al. (2009) suggest that the method is highly suitable as the principal leachate treatment option given that it is highly resilient and little affected by the fluctuations in temperature, flow rate and loading associated with effluents. The advantage further lies with the ability of the system to remove a wide range of critical pollutants at a minimum possible investment and operating cost, making it feasible in places where finance and technological constraints are a consideration. The only operating cost is associated with electricity because sufficient power input is required to diffuse enough oxygen into the leachate. In terms of design and facility, this experiment resembles facultative lagoons that are simple enough to provide a sufficient space for leachate retention, aeration and settling solids. Several lagoons or basins resembling this study (facultative type) were developed in Italy, Britain, Ireland and the UK (Frascari et al., 2004). Generally, they are located on a clay declivity and also surrounded by a 0-2m thick layer of more permeable clay filling. They have several common features: high density poly-ethylene lining and few aerators; automatic timers, sensors, switches; and a provision for leachate collection. While the capacity of the lagoon varies from place to place, for a landfill receiving small waste input (50 tonnes per day) at a place receiving an average annual rainfall of 1200 mm, a capacity of 1000 m3 is suitable. This capacity allows a required retention time of 10 days at a maximum design flow rate of about 100 m3 per day (Robinson & Grantham, 1988). 83 Given Bhutan’s SWM background, that is a similar waste input rate (64.5 tonnes per day in urban Bhutan), less per capita waste generation (0.5 kg/day (rural/urban), the organic nature of the waste (60% organic fraction) and similar climatic conditions, an aerobic treatment system is highly recommendable and feasible for Bhutan. 6.1.8 Drawbacks of the experiment Although both bench scale and onsite aerobic treatment studies came to a general consensus that the system is little affected by the fluctuations in temperature, most results have been reported from experiments performed at room temperature, generally stated to be about 20-30oC. Treatment systems carried out at 3oC, 10oC and 15oC also achieved significant outcomes, but what is not known is the effect of extreme low temperatures (below 0oC) on treatment efficiency. Most urban places in Bhutan have an extreme winter season where the mean temperatures fall to ¯8oC in winter. Robinson & Grantham (1988) reported a case in mid-Wales, UK, where the lagoons froze forming ice several inches thick at 2-3oC. Though, it was stated that there was no noticeable effect on the effluent quality when the situation occurred, such cases might result in other unforeseen operation problems. Likewise, simple aeration operation style is possibly associated with sludge bulking and scum formation. Boyle & Ham (1974) reported that a treatment unit with one hour settling gave rise to sludge problems resulting in high effluent solid concentration. This requires a separate collection and disposal of settled sludge in the landfill. In the present experiment, sludge build up was negligible and scum formation was not evident by visual assessment of the two units, apparently because bench scale experiments involved lesser amounts of leachate. Since the treatment units in the above study were not “fill and draw” systems, there is a lack of information on the influent and effluent flow rates. This might give rise to overflow of leachate, especially in the monsoon season when the rate of leachate generation in the landfill rises. In practice, leachates have to be treated in continuous flow rather than the batch mode system (Matthews et al., 2009). 84 6.2 Column Experiments In total nine different characteristics of a typical leachate were discussed to determine the suitability of filter materials in a leachate treatment. The results varied from each filter material to the other; nevertheless a common observation is that all the materials were highly recommendable for removal of SRP from leachate. Apart from that, the materials have a considerable effect in reducing suspended and dissolved solids as indicated by the slight decrease in turbidity and EC. 6.2.1 P-removal Results of P-removal efficiency obtained from the column experiment were then compared with the batch experiment results. The P-removal efficiencies in the columns were highest for BFS (92%) followed by GAC (68%) and sand (40%). As anticipated, the batch experiments also confirmed that GAC and BFS could remove phosphorus more significantly than sand. That is BFS has the highest P-sorption capacity with an ability to adsorb 257 mg of phosphorus in every one kg of filter material. Equally efficient is the GAC with a P-sorption capacity estimated at 249 mg of phosphorus in every one kg. Sand exhibited a capacity to remove 27 mg of phosphorus in every one kg and thus will result in a lower P-removal efficiency. The fact that both the experiments demonstrated BFS can remove phosphorus much better than the GAC and sand, was supported by Johansson (1999), who reported that BFS can remove more than 90% phosphorus from a P-solution graded 10 mg/L. With respect to GAC, the material is known for its effectiveness in treating contaminated wastewater and leachate, particularly the removal of phosphorus. This could be attributed to the fact that GAC has a high surface area to volume/weight ratio than most filter materials. Figure 5.10 exhibits that GAC has an approximate effective particle size (D10) equivalent to 2.8 mm, which is much higher than BFS (0.5 mm) and sand (0.25 mm). In addition, the batch experiment results confirm P-sorption as one main factor behind P-removal from the leachate. However, P-removal efficiencies obtained from column 85 filtration will be less than those obtained in batch experiments because some P-leaching may always occur (Kuryer et al, 1995; cited in Kim, 1999). Similar to P-removal, the variation in overall effect of the filter materials on the leachate quality can be attributed to numerous controlling factors. Firstly, treatment of leachate by the column filtration used three materials with large differences in their physical and chemical properties, the details of which are discussed in the following sections. Secondly, leachate is a complex mixture of organic and inorganic substances capable of undergoing various physical and chemical reactions. 6.2.2 The effect of chemical composition The need to understand a filter material by its chemical composition is fundamental because each filter material is different on its own. BFS is essentially composed of silicates and alumino-silicates of Ca. Sand is roughly similar to BFS and contains SiO2 as the main component followed by oxides of Al, K and Fe (Kietlinska & Renman, 2005). On the other hand, GAC is an entirely different material containing carbon as the main component. Thus, when a leachate is permeated through them, complex physical and bio-chemical processes are anticipated eventually resulting in a change in effluent quality. Since removal of phosphorus predominantly takes place by several mechanisms: rapid removal or adsorption; chemical precipitation; and ion exchange (Aulenbach & Meisheng, 1988; Johansson, 1999; Oguz, 2004), the chemical composition of the filter material will have a significant impact on P-removal from the leachate. BFS mainly contains metals like Ca, Al, K and Fe in the form of their oxides. Their presence favours physical sorption of SRP or ortho-phosphate ions on the surface of the material particles. In addition, the respective metal ions can help remove phosphorus by precipitation, which occurs by transforming the soluble phosphate in the influent to relatively insoluble metal phosphates (Ca, Al, K and Fe), particularly when the effluent is alkaline. 86 Similarly, partial dissolution and subsequent hydrolysis of Ca and alumina silicates from the slag allows adsorption of other metal ions (Copper (Cu), Ni and Zn) on its surface either by exchange or replacement when releasing Ca ions into the solution. (Dimitrova & Mehanjiev, 1999). Most researchers have in fact proposed precipitation of any of Ca- phosphates as one mechanism for P-removal. 6.2.3 Contact time & hydraulic conductivity Another explanation for observed differences between the P-removal efficiencies among different filter materials is the variation in contact times, which in turn is induced by varying hydraulic conductivity values. It is observed that the materials with lower hydraulic conductivity ensure greater contact time and thus promote P-sorption mechanisms to a greater extent than with shorter contact times and higher hydraulic conductivity. As a result, hydraulic conductivity is suggested as the most conceivable explanation for the observed differences in P-removal between GAC, BFS and sand. The BFS column, which achieved the highest P-removal efficiency (92%), had the lowest hydraulic conductivity (29 m/day). That is, the lower hydraulic conductivity allows for more opportunities for leachate to come into contact with a significant volume of filter materials. Inversely, although GAC has a better ability to adsorb phosphorus like BFS (Figure 5.11), it resulted in a comparatively lower P-removal efficiency (68%), mainly due to the shorter contact times induced by its high hydraulic conductivity (192 m/day). However, in the case of sand, low hydraulic conductivity (32 m/day) and high contact time did not improve its ability to remove phosphorus, possibly owing to its chemical composition. Hydraulic conductivity (m/day) = GAC (192) > sand (32) > BFS (29) Approximate contact time (minutes) for 500 mL of influent in column configuration used = BFS (14) > sand (12) > GAC (3) Similar conclusions were also observed by Nilsson (1990; cited in Johansson, 1999). Therefore the effectiveness of P-removal is higher for filter materials with lower hydraulic conductivity and better contact times. 87 6.2.4 Longevity According to Johansson (1999), longevity of the filter material is an important practical factor to take into consideration prior to its use in onsite treatment systems, which use filter materials such as sand or soil. Longevity is limited by factors such as saturation, hence, once the material attains a saturation point; it is no longer effective for further treatment. Considering all other factors constant, longevity may be defined as a point of time, where the material no longer significantly influences the quality of the effluent. In this experiment, the saturation point of all the filter materials is achieved around the 2nd run, corresponding to a contact time of 15 minutes for GAC, and approximately an hour for both BFS and sand. This is assumed from that fact that after the 2nd run, the effluent leachate quality remained steady for most pollutant parameters, especially phosphorus, turbidity, EC and total alkalinity (See Figures 5.11, 5.13, 5.15 and 5.16, respectively). This implies that different filter materials will have different durabilities in the treatment industry. 6.2.5 Particle size distribution, porosity percentage (%) and bulk density The particle size distribution of a filter material is yet another critical factor in defining the varying performance of a filter material because adsorption is one main mechanism by which the filter materials adsorb or collect molecules of dissolved compounds on the surface of an adsorbent solid. Adsorption is essentially a surface phenomenon (Brady & Weil, 1996) occurring as a result of weak physical interaction between the surface of adsorbent and the metallic salts of phosphate (Oguz, 2004). Considering the above assumptions, filter materials with smaller particle size will have greater advantage over the ones that are larger. This is because smaller particle size will have larger surface area available for adsorption in a given volume and ensures greater interaction with the contaminants present in leachate. While GAC has larger particle size ( effective size (D10) of approximately 2.8 mm) and smaller surface area available for greater interactions, each particle has a honeycomb fabric with very high surface area-hence its effectiveness in capturing contaminants. This leaves the material with comparatively higher ability to remove phosphorus even at a very high hydraulic conductivity. Better results may be achievable by mixing peat with GAC in the filter matrix to reduce the hydraulic conductivity (Johansson, 1999). BFS has similar 88 properties with a D10 value of 0.5 mm. Although sand has a smaller particle size (D10 <0.25 mm), it do not have internal surfaces.Thus it has low effectiveness in capturing contaminants. Again, particle size may be related to two other factors, porosity and bulk density. That is the bigger the particle size, the higher the proportion of pore space to solid per unit volume (Brady & Weil, 1996) and the lower the bulk density and vice versa. The inverse relation between estimated porosity percent (%) and bulk density is shown below: Porosity % = GAC (68%)>BFS (40%)>Sand (36%) Bulk Density = (kg/m3) GAC (300) <BFS (1500) <Sand (1600) The significance of low porosity is that it allows reduced infiltration of the influent sample or water and thus allows very low interaction between the filter materials and the influent. This might be one possible reason behind the low performance of sand in treating leachate. In addition to particle size, porosity, and bulk density, the structure of the filter material is likely to result in differential removal of phosphorus. For example, Johansson (1999) reported that P-removal efficiencies of BFS differed even when the structure of the filter changed. That is, two different surface structures (amorphous and crystalline) of the same filter material (BFS) having the same particle size (0.25 mm - 4 mm) exhibited differential P-removal efficiency. Amorphous slag absorbed phosphorus to a greater extent than crystalline slag owing to its vitreous surface structure that allows large amounts of phosphate ions from the solution to penetrate between the filter particles in a shorter period of contact time than those with fine crystalline particles. This phenomenon partially explains why sand does not have an ability to remove P like GAC and BFS, even though it has a similar hydraulic conductivity like BFS. 6.2.6 Drawbacks of the experiment Since BFS contains a high percentage (%) of Ca in the form of CaO (35%), it is likely that Ca can react with sulphate commonly found in leachate to create gypsum (Kietlinska & Renman, 2005). Therefore, there is a need for an addition of organic 89 component (peat) to the filter matrix to decrease this effect. Peat is also known to decrease the hydraulic conductivity of the coarse BFS materials and also act as an adsorbent for heavy metals. Filter materials might result in leaching several elements. Kietlinska & Renman (2005) earlier demonstrated that BFS and sand release about 200 mg/L and 500 mg/L of Al into the leachate. The authors attributed such occurrence to different chemical reactions such as ion exchange and mineralisation of organic materials with strong leachate. Finally, the experimental results with landfill leachate are more difficult to interpret than those performed with aqueous solutions. The differences in the results obtained by the investigation of reactive filter materials arise from the fact that leachate is a chemically complex liquid. 90 Chapter 7 Conclusions and Recommendations 7.1 Laboratory Aeration Experiments The major conclusions from the laboratory experiments are that the aerobic treatments were successful in effectively treating raw landfill leachate and in reducing concentrations of certain pollutants. Leachate was effectively stabilised at a room temperature (21oC) without any pH adjustment or nutrient addition. The quality improved significantly with 100% ammonia-nitrogen and 96% turbidity reduction at a retention time of approximately 16 days. Likewise, the leachate quality improved aesthetically both in terms of colour and odour. Such changes demonstrate that a medium strength leachate can be treated by a simple aerobic process without involving complex processes and technology. On the other hand, in the absence of aeration, there was no significant improvement in leachate quality, although ammonia and turbidity reduced by 29% and 46%, respectively. The slight reduction in ammonia-nitrogen and turbidity was attributed to the biodegradation of organics present in the leachate in the presence of available dissolved oxygen and volatilisation of ammonia to the atmosphere. However, over this time, there was no improvement in the quality. Instead, the leachate samples began to impart a foul odour with an increasing retention time reflecting a trend toward anoxic conditions. 7.2 Laboratory Column Experiments With respect to column experiments, out of the nine different characteristics discussed, the filter materials were most effective at removing phosphorus from the leachate. There was a significant decrease in the P-concentration of the leachate samples after being filtered through the respective materials. That is P-concentration decreased by approximately 68%, 92% and 40%, (initial~25 mg/L phosphorus) for GAC, BFS and sand, respectively. This occurred at a corresponding contact time of approximately 30 minutes for GAC, two and half hours for BFS and two hours for sand. By comparison, GAC and BFS were more suitable for P-removal than sand. Apart from significant Premoval, the filter materials appeared to have absorbed other forms of both organic and 91 inorganic pollutants as reflected by a considerable decrease in turbidity (with an exception for GAC) and electrical conductivity. Overall, an important observation is that the two treatment experiments resulted in significant differences based on their ability to treat landfill leachate. While the aerobic treatment is most effective and suitable for treating OM, particularly ammonia, the filter materials are suitable for removing SRP. On this note, it may be suggested that the two treatment experiments can together make a unique or effective combination to form an independent leachate treatment train. In the train system, the main concept, aerobic treatment, will function better as a primary clarifier system to remove organic materials and ammonia first and then allow the resulting pollutants (phosphorus, metals and refractory solids) to be removed by passing the leachate through a filter bed. Implementation of this system would help in accomplishing complete or better effluent quality that may not be possible using either of the processes separately. 7.3 Leachate Characterisation Firstly, the statistical evaluation of the 14 year long term leachate monitoring data revealed that the pattern of waste decomposition in SWMC landfill is roughly similar to other landfills. Leachate generated from this landfill has high concentrations of OM, ammonia-nitrogen, sodium and chlorides. These contaminants increase the pollution potential of the leachate making it unsafe for direct release into the nearby surface water or environment. Secondly, a closer investigation of the change in leachate characteristics illustrated the temporal variations of the landfill. In 14 years, the landfill showed approximately two distinct phases. The first phase lasted for a period of first four years (1995-1999) and included the initial stabilisation period (first year), which is the time taken by the waste to start decomposition and for leachate to be generated. Leachates formed during this period were essentially pH neutral, of low toxicity and characterised by very low concentrations such as ammonia-nitrogen, electrical conductivity, suspended solids and metals (Na, K and Fe). The concentrations of, BOD, COD and TOC were however high. With the onset of the second phase (2000-2009), leachates became slightly alkaline and 92 more toxic. Very high concentrations of the above pollutants were then recorded in the leachate samples. Finally, the relation between rainfall and leachate production from the landfill for the past 14 years helped in estimating the approximate volume of leachate generation. The prediction is that for a mean annual rainfall of 1165 mm/year, the leachate produced was up to 400 mm/year. This roughly indicates leachate percolation was up to 35% of the rainfall and falls within the range (15-50%) reported by Canziani & Cossu (1989; cited in Tatsi & Zoubolis, 2002). 7.4 Solid Waste Management Options for Bhutan Currently, land filling of MSW is the primary method of solid waste disposal in Bhutan. Therefore, low cost leachate treatment methods are necessary to reduce the contamination of landfill leachate on the environment. When leachate treatment is still not feasible, composting may be adopted as an alternative waste management technique. After all the waste generation trend indicates a fourfold increase in waste generation for the urban centres of Bhutan by 2020. Therefore, a sustainable and cost effective option is also necessary to overcome what may prove to be a significant environmental management issue for the country. Likewise, this thesis has indentified the main problems of SWM in Bhutan based on secondary evaluation of the performance of SWM systems in the country. The evaluation, which is based on earlier research carried out in Bhutan, suggested that unlike other countries, there is a lack of proper sorting and segregation of waste at the source. Different types of waste were all collected together and transported to the landfills. It is also clear that most landfills in Bhutan are mere open dumps or low lying depressions on the ground and thus do not have a proper design and structure nor a provision for leachate and gas collection facilities. 93 7.5 Recommendations and Further Research The following recommendations are based on the results of the laboratory experiments, a review of the literature on SWM in various countries and prevailing low cost leachate treatment options. Although the laboratory experiments were highly convincing in demonstrating that leachate treatment is possible both through aerobic treatment and media filtration, it is very important to remember that the results obtained from this study are achieved in the laboratory and at a bench scale. The conditions prevailing here might differ from those occurring in the field or on-site. With this qualification, the laboratory results indicate what could be expected in the field. Further research is therefore suggested at the field scale to increase the knowledge of using the filter materials or aeration in order to implement it successfully. The analysis of various research on SWM in Bhutan points out that SWM systems in Bhutan have existed only for a shorter period of time as compared to Australia and some other countries in the region. Even though collection, transportation and disposal of waste are prevalent in major urban cities in Bhutan, all the three components of an integrated waste management system such as infrastructure and maintenance are lacking there. 94 References Aghamohammadi, N., Aziz, H.A., Isa, M.H. and Zinatizadeh, A.A. (2007). Powdered activated carbon augmented activated sludge process for treatment of semi-aerobic landfill leachate using response surface methodology. Tehran, Iran. Journal of Bioresource Technology, 98, 3570–3578. Allison, E. (2008). The dark side of light: Managing non-biodegradable wastes in Bhutan's rural area. Journal of Mountain Research and Development, 28, No. 3/4, 205-209. Al-Yaquot, A.F., Koushki and Hamoda, M.F. (2002). Public opinion and siting solid waste landfills in Kuwait. Safat, Kuwait. Journal of Resources, Conservation and Recycling, 35, 215– 227. Amokrane, A., Comel, C. and Veroni, J. (1997). Landfill leachate pre-treatment by coagulationflocculation. Cedex, France. Journal of Water Research, 31, No, 11, 775 -2782. Arceivala, S. J. (1973). Simple waste treatment methods- Aerated lagoons, oxidation ditches, stabilisation ponds in warm and temperate climates. Published by Middle East Technical University. Ankara, Turkey. Aulenbach, D. B. and Meisheng, N. (1988). Studies on the mechanism of Phosphorous removal from treated waste water by sand. Journal of Water Pollution Control Federation, 60, 2089-2093. Australian and New Zealand Environment and Conservation Council. (1992). Australian water quality guidelines for fresh and marine waters. Australia. Autret, E., Berthier, F., Luszezanec, A. and Nicolas, F. (2007). Incineration of municipal and assimilated wastes in France: Assessment of latest energy and material recovery performances. Cedex, France. Journal of Hazardous Materials, B139, 569-574. Ayomoh, M.K.O., Oke, S. A., Adedeji, W. O. and Charles-Owaba, O. E. (2008). An approach to tackling the environmental and health impacts of municipal solid waste disposal in developing countries. Lagos, Nigeria. Journal of Environmental Management, 88, 108– 114. Aziz, H. A., Alias, S., Adlan, M.N, Faridah, Asaari, A. H. and Zahari, M.S. (2007). Colour removal from landfill leachate by coagulation and flocculation processes. Penang, Malaysia. Journal of Bioresource Technology, 98, 218-220. Baird, C. (1999). Environmental Chemistry. 2nd Edition. WH Freeman and Company, New York 2000. Berge, N.D., Reinhart, D.R., Dietz, J. and Townsend, T. (2006). In-situ ammonia removal in bioreactor landfill leachate. Orlando, USA. Journal of Waste Management, 26, 334-343. 95 Berge, N.D., Reinhart, D.R., Dietz, J. and Townsend, T. (2007). The impact of temperature and gas-phase oxygen on kinetics of in situ ammonia removal in bioreactor landfill leachate. Massachusetts, USA. Journal of Water Research, 41, 19071914. Boyle, W.C. and Ham, R.K. (1974). Biological treatability of landfill leachate. Journal of Water Pollution Control Federation, 46, No. 5, 860-872. Brady, N.C. & Weil, R.R. (1996). The nature and properties of soil. 11th edition. Prentice- Hall, Inc. A Sina and Schuster Company. New Jersey 07458. Castrillon, L., Fernandez-Nava, Y., Ulmanu, M., Anger, I. and Maranon, E. (2010). Physicochemical and biological treatment of MSW landfill leachate. Gijon, Spain. Journal of Waste Management, 30, 228-235. Carbtrol Corporation (1992). Granular activated carbon for water and wastewater treatment. Retrieved 15/01/2011 from http://www.carbtrol.com/water&waste.pdf Cecen, F. & Aktas, O. (2001). Addition of activated carbon to batch activated sludge reactors in the treatment of landfill leachate and domestic wastewater. Bogazici University, Institute of Environmental Sciences, 80815, Bebek, Istanbul, Turkey. Journal of Chemical Technology and Biotechnology, 76, 793-802. Cecen, F., Erdincler, A. and Kilic, E. (2003). Effect of powdered activated carbon addition on sludge dewaterability and substrate removal in landfill leachate treatment. Istanbul, Turkey. Journal of Advances in Environmental Research, 7, 707-713. Chen, P.H. (1996). Assessment of leachates from sanitary landfills: Impact of age, rainfall, and treatment. Taichung, Taiwan. Journal of Environment International, 22, No. 2, 225237. Christensen, T.H., Cossu, R. and Stegmann, R. (1992). Land filling of waste: Leachate. Elsevier Science Publishers Ltd. England. Christensen, T.H., Kjeldsen, P., Bjerg, P.L., Jense, D.L., Christensen, J.B., Baun, A., Albrechtsen, H. and Heron, G. (2001). Review- Biochemistry of landfill leachate plumes. Lyngby, Denmark. Journal of Applied Geochemistry, 16, 659-718. Climate data of Bhutan (2009). Hydro-Met Services Division, Department of Energy, Ministry of Economic Affairs, Bhutan. Cook, E. N. and Foree, E. G. (1974). Aerobic biostabilisation of sanitary landfill leachate. Journal of Water Environment Federation, 46, No. 2, 380-392. Country report on the forest resources of Bhutan (2000). Forestry Department, Food and Agriculture Organization of the United Nations. 96 Dimitrova, S. V. and Mehanjiev, D. R. (1999). Interaction of Blast Furnace Slag with heavy metal ions in water solutions. Journal of Water Resources, 34, No. 6, 1957-1961. Duggan, J. (2005). The potential for landfill leachate treatment using willows in the UK—A critical review. Northampton, UK. Journal of Resources, Conservation and Recycling, 45, 97– 113. Eaton, A.D., Clesceri, L.S. & Greenberg, A.E. (1995). Standard methods for the examination of water and wastewater. 19th edition. American Public Health Association. 1015 fifteenth Street, NW, Washington, DC 2005. El-Fadel, M., Findikakis, A.N. and Leckie, J.O. (1997). Environmental impacts of solid waste land filling. California, USA. Journal of Environmental Management, 50, 1-25 Ernst, A.-A. (1990). A review of solid waste management by composting in Europe. Duisburg, Germany. Journal of Resources, Conservation and Recycling, 4, 135-149. Farrell, M., and Jones, D. L. (2009). Critical evaluation of municipal solid waste composting and potential compost markets. Gwynedd, UK. Journal of Bioresource Technology, 100, 4301-4310. Foo, K.Y. and Hameed, B.H. (2009). An overview of landfill leachate treatment via activated carbon adsorption process. Penang, Malaysia. Journal of Hazardous Materials, 171, 54–60. Frascari, D., Bronzini, F., Giordano, G., Tedioli, G. and Nocentini, M. (2004). Long-term characterization, lagoon treatment and migration potential of landfill leachate: a case study in an active Italian landfill. Bologna, Italy. Journal of Chemosphere, 54, 335-343. Gourdon, R., Comel, C., Vermande, P. and Veron, J. (1989). Fractionation of the organic matter of a landfill leachate before and after aerobic or anaerobic biological treatment. Cedex, France. Journal of Water Resources, 23, No. 2, 167-173. Hamoda, M. F., AbuQdais, H. A. and Newham, J. (1998). Evaluation of municipal solid waste composting kinetics. Safat, Kuwait. Journal of Resources, Conservation and Recycling, 23, 209–223. Henry, R.K., Yongsheng, Z. and Jun, D. (2006). Country Report: Municipal solid waste management challenges in developing countries – Kenyan case study. Changchun, China. Journal of Waste Management, 26, 92–100. Hunsicker, M.D., Crockett, T.R. and Labode, B.M.A. (1996). An overview of the municipal waste incineration industry in Asia and the former Soviet Union. Omaha, USA. Journal of Hazardous Materials, 47, 31-42. Johansson, L. (1999). Blast Furnace Slag as phosphorus sorbents-column studies. The Science of the Total Environment, 229, 89-97. 97 Johnke, B. (1992). Waste Incineration- An important element of the integrated waste management system in Germany. Berlin, Germany. Journal of Waste Management, 10,303-315 Joos, W., Carabias, V., Winistoerfer, H. and Stuecheli, A. (1999). Social aspects of public waste management in Switzerland. Winterthur, Switzerland. Journal of Waste Management, 19, 417-425. Jun, D., Yonsheng, Z., Weihong, Z. & Mei, H. (2009). Laboratory study on sequenced permeable reactive barrier remediation for landfill leachate-contaminated groundwater. Jilin, China. Journal of Hazardous Materials, 161, 224–230. Kargi, F. and Pamukoglu, M.Y. (2004). Repeated fed-batch biological treatment of pre-treated landfill leachate by powdered activated carbon addition. Izmir, Turkey. Journal of Enzyme and Microbial Technology, 34, 422–428. Kietlinska, A. and Renman, G. (2005). An evaluation of reactive filter media for treating landfill leachate. Stockholm, Sweden. Journal of Chemosphere, 61, 933-940. Kim, S. (1999). The impact of biomass harvesting on Phosphorus uptake by emergent wetland plants. M sc. Thesis. The University of Newcastle, Australia. Knox, K. (1983). Treatability studies on leachate from a co-disposal landfill. Cleanaway Limited, Claydons Lane, Raylegh, Essex SS6 7UW, Great Britain. Journal of Environmental Pollution (Series B), 5, 157-174. Knox, K. (1985). Leachate treatment with nitrification of ammonia. Essex, England. Journal of Water Resource, 19, No. 7, 895-904. Larsen, L. and Borrild, K. (1991). Waste management in Copenhagen: Principles and trends. Copenhagen, Denmark. Journal of Waste Management & Research, 9, 239-258. Li, W., Hua, T., Zhou, Q., Zhang, S. and Li, F. (2010). Treatment of stabilized landfill leachate by the combined process of coagulation/flocculation and powder activated carbon adsorption. Journal of Desalination. Lim, Y. N., Shaaban, M. G. and Yin, C. Y. (2009). Treatment of landfill leachate using palm shellactivated carbon column: Axial dispersion modelling and treatment profile. Kuala Lumpur, Malaysia. Journal of Chemical Engineering, 146, 86-89. López, M., Soliva, M., Martínez-Farré, F. X., Fernández, M. and Huerta-Pujol, O. (2010). Evaluation of MSW organic fraction for composting: Separate collection or mechanical sorting. Barcelona, Spain. Journal of Resources, Conservation and Recycling, 54, 222228. Lucas, S.A. (2007). Temporal sodium flux in a woodlot soil irrigated with secondary treated effluent: The implications for sustainable irrigation and soil management. Unpublished PhD thesis. School of Environmental and Life Sciences. The University of Newcastle, NSW. Australia. 98 McArdle, J.L., Arozarena, M.M. & Gallagher, W.E. (1988). Treatment of hazardous waste leachate- Unit operations and costs. Noyes Data Corporation, Park Ridge, New Jersey, USA. Matthews, R., Winson, M. and Scullion, J. (2009). Treating landfill leachate using passive aeration trickling filters; effects of leachate characteristics and temperature on rates and process dynamics. Journal of Science of the Total Environment, 407, 2557–2564. Maranon, E., Castrillon, L., Fernandez-Nava, Y., Fernandez-Mendez, A. and Fernandez-Sanchez, A. (2008), Coagulation–flocculation as a pre-treatment process at a landfill leachate nitrification–denitrification plant. Gijon, Spain. Journal of Hazardous Materials, 156, 538–544. Mehmood, M.K., Adetutu, E., Nedwell, D.B. and Ball, A.S. (2009). In-situ microbial treatment of landfill leachate using aerated lagoons. Adelaide, South Australia. Journal of Bioresource Technology, 100, 2741-2744. Mendes, M.R., Aramaki, T. and Hanaki, K. (2004). Comparison of the environmental impact of incineration and land filling in São Paulo City as determined by LCA. Tokyo, Japan. Journal of Resources, Conservation and Recycling, 41, 47–63. Mohan, S. and Gandhimathi, R. (2009). Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. Chennai, India. Journal of Hazardous Materials, 169,351-359. Ngoc, U.N. and Schnitzer, H. (2009). Sustainable solutions for solid waste management in Southeast Asian countries. Graz, Austria. Journal of Waste Management, 29, 1982– 1995. Nehrenheim, E., Waara, S. and Johansson, L. W. (2008). Metal retention on pine bark and blast furnace slag – On-site experiment for treatment of low strength landfill leachate. Bioresource Technology, 99, 998-1005. New South Wales climate data (1995-2009). Bureau of Meteorology (BOM), Australia. Retrieved 01/08/2010 from http://www.bom.gov.au/climate/averages/tables/cw_061078.shtml. Nivala, J., Hoos, M. B., Cross, C., Wallace, S. and Parkin, G. (2007). Treatment of landfill leachate using an aerated, horizontal subsurface-flow constructed wetland. Minnesota, USA. Journal of Science of the Total environment, 380, 19-27. Nora, K. (2007). Assessment of aeration and leachate recirculation in open cell landfill operation with leachate management strategies. Master of Engineering in Environmental Engineering and Management Thesis. School of Environment, Resources and Development. Asian institute of Technology, Thailand. 99 Ntampou, X., Zoubolis, A.I. and Samaras, P. (2006). Appropriate combination of physico-chemical methods (coagulation/flocculation and ozonation) for the efficient treatment of landfill leachates. Thessaloniki, Greece. Journal of Chemosphere, 62,722-730. Oakes, P. (2009). Municipal solid waste processing. An overview of MSW composting processes and projects in Australia, including basic funding structures used. Australia. Journal of Biocycle International. Oguz, E. (2004). Removal of phosphate from aqueous solution with blast furnace slag. Journal of Hazardous Materials, B114, 131-137. Patterson, R.A. and Jones, M.J. (2001). Proceedings of on-site’ 01 Conference. Advancing onsite wastewater systems. University of New England. Penjor, Y. (2008). Wastes management for Bhutanese happiness: Priority should be public awareness and education. National Environment Commission (NEC), Thimphu, Bhutan. Phuntsho, S., Dulal, I.,Yangden, D., Tenzin., U.M., Herat, S., Shon, H. and Vigneswaran, S. (2010). Studying municipal solid waste and composition in the urban areas of Bhutan. Waste Management and Research, 28, No.6, 545-551. Pivato, A. and Gaspari, L. (2006). Acute toxicity test of leachate from traditional and sustainable landfills using luminescent bacteria. Padova, Italy. Journal of Waste Management, 26, 1148-1155. Read, A.D., Hudgins, M., Harper, S., Phillips, P. and Morris, J. (2001). The successful demonstration of aerobic land filling: The potential for a more sustainable solid waste management approach? Northampton, UK. Journal of Resources Conservation & Recycling, 32, 115-146. Renou, S., Givaudan, J.G., Poulain, S., Dirassouyan, F. and Moulin, P. (2008). Landfill leachate treatment: Review and opportunity. Cedex, France. Journal of Hazardous Materials, 150, 468-493. Rivas, F.J., Beltran, F.J., Gimeno, O., Frades, J. and Carvalho, F. (2006). Adsorption of landfill leachates onto activated carbon- Equilibrium and Kinetics. Badajoz, Spain. Journal of Hazardous Materials, B131, 170-178. Robinson, H.D, Knox, K., Bone, B.D. and Picken, A. (2005). Leachate quality from land filled MBT waste. Shropshire, UK. Journal of Waste Management. 25, 383-391. Robinson, H. D. and Grantham, G. (1988). The treatment of landfill leachates in on-site aerated lagoon plants: experience in Britain and Ireland. Shrewsbury, England. Journal of Water Resources. 22, No. 6, 733-747. Robinson, H. D. and Maris, P. J. (1983). The treatment of leachates from domestic wastes in landfills- I -Aerobic biological treatment of medium strength leachate. Stevenage, England. Journal of Water Resources, 17, No. 11, 1537-1548. 100 Rushbrook, P.E. and Finnecy, E.E. (1988). Planning for future waste management operations in developing countries. Journal of Waste Management & Research, 6, 1-21. Schmidt, A. T. (2005). Port Stephens Council: Reaping the rewards of ‘Best Practice’ waste management. NSW, Australia. Journal of Waste Disposal and Water Management. Sedlak, R. (1991). Phosphorus and nitrogen removal from municipal waste water: Principles and Practices. 2nd Edition. Lewis Publishers, USA. Shao, L.M., He, P., and Li, G. (2008). In situ nitrogen removal from leachate by bioreactor landfill with limited aeration. Shanghai, China. Journal of Waste Management, 28, 1000–1007. Shou-liang, H., Bei-dou, X., Hai-chan, Y., Shi-lei, F., Jing S. and Hong-liang, L. (2008). In situ simultaneous organics and nitrogen removal from recycled landfill leachate using an anaerobic-aerobic process. Beijing, China. Journal of Bioresource Technology, 99, 6456-6463. Shiralipour, A., McConnell, D. B. and Smith, W. H. (1992). Uses and benefits of MSW compost: A review and an assessment. Florida, USA. Journal of Biomass and Bioenergy, 3, Nos 34, 267-279. Shiskowski, D.M. and Mavinic, D.S. (1997). Biological treatment of a high ammonia leachate: Influence of external carbon during initial start-up. BC, Canada. Journal of Water Resources, 32, No.8, 2533-2541. Slater, R. A. and Frederickson, J. (2001). Composting municipal waste in the UK: some lessons from Europe. Milton Keynes, UK. Journal of Resources Conservation and Recycling, 32, 359-374. Summerhill Waste Management Centre (2002). Annual Report 2001/2002, Newcastle, New South Wales. Australia. Tatsi, A.A. and Zoubolis, A.I. (2002). A field investigation of the quantity and quality of leachate from a municipal solid waste landfill in a Mediterranean climate (Thessaloniki, Greece). Thessaloniki, Greece. Journal of Advances in Environmental Research, 6, 207-219. Tatsi, A.A., Zoubolis, A.I., Matis, K.A. & Samaras, P. (2003). Coagulation–flocculation pretreatment of sanitary landfill leachates. Thessaloniki, Greece. Journal of Chemosphere, 53, 737-744. Tchobanoglous, G. & Kreith, F. (2002). Hand Book of Solid Waste Management. 2nd edition. McGraw-Hill Publishing Company, New York, USA. Themelis, N.J. and Ulloa, P.A. (2007). Methane generation in landfills. New York, USA. Journal of Renewable Energy, 32, 1243-1257. 101 Tyrrel, S.F., Leeds-Harrison, P.B. and Harrison, K.S. (2002). Removal of ammoniacal nitrogen from landfill leachate by irrigation onto vegetated treatment planes. Bedfordshire, UK. Journal of Water Research, 36, 291-299. Tzoupanos, N. D., Zoubolis, A. I. and Zhao, Y.-C. (2008).The application of novel coagulant reagent (polyaluminium silicate chloride) for the post-treatment of landfill leachates. Journal of Chemosphere, 23, 729-736. Uddin, S.N., Taplin, R. and Yu, X. (2007). Energy, environment and development in Bhutan. Sydney, Australia. Journal of Renewable & Sustainable Energy Reviews, 11, 20832103. Wang, Q., Matsufuji, Y., Dong, L., Huang, Q., Hirano, F. and Tanaka, A. (2006). Research on leachate recirculation from different types of landfills. Japan. Journal of Waste Management, 26, 815-824. Wang, Z., Huang, L., Feng, X., Xie, P. and Liu, Z. (2010). Removal of phosphorus in municipal landfill leachate by photochemical oxidation combined with ferrate pre-treatment. Wuhan, China. Journal of Desalination and Water Treatment, 22, 111-116. Wang, S., Wu, X., Wang, Y. Li, Q. and Tao, M. (2008). Removal of organic matter and ammonia nitrogen from landfill leachate by ultrasound. Wuhan, China. Journal of Ultrasonics Sonochemistry, 15, 933-937. Wei, Y.-S., Fan, Y.-B., Wang, Y.-B. M.-J. and Wang, J.-S. (2000). Composting and compost application in China. Beijing, China. Journal of Resources, Conservation and Recycling, 30, 277-300. Wei, Z., Xi, B., Y., Z., S., W., Liu, H. and Jiang, Y. (2007). Effect of inoculating microbes in municipal solid waste composting on characteristics of humic acid. Journal of Chemosphere, 68, 368-374. Whitehead, J.H., Geary, P.M. & Kidd, R.W. (2000). Advances in Environmental Management in the Hunter Region. Hunter Environmental Institute. Published by Conference Publications, PO Box 107. Springwood, NSW 2777, Australia. Yahmed, A.B., Saidi, N., Trabelsi, I., Murano, F., Dhaifallah, T., Bousselmi, L. and Ghrabi, A. (2009). Microbial characterization during aerobic biological treatment of landfill leachate (Tunisa). Soliman, Tunisa. Journal of Desalination, 246, 378-388. Yamamura, K. (1983). Current status of solid waste management in Japan. Journal of Waste Management and Research, 1, 1-15. Yang, K., Zhou, X., Yan, W., Hang, D. and Steinmann, P. (2008). Landfills in Jiangsu province, China, and potential threats to public health: Leachate appraisal and spatial analysis using geographic information system and remote sensing. Wuxi, China. Journal of Waste Management, 28, 2750-2757. 102 Appendix A 1) Aerobic Treatment of Leachate (Control and Experiment) over a period of 20 days. Number of Days Parameters Initial 2 Dark Brown Dark Brown with greenish layer formation at the top. Dark Brown Turbidity (NTU) PH Electrical conductivity (dS/m) Alkalinity ( mg/L as CaCO3 ) DO Ammonia-nitrogen Nitrite-nitrogen Nitrate-nitrogen Soluble reactive phosphorus 110 8.2 100 8.8 6.64 Temperature (oC) Colour 4 6 12 14 16 18 20 Dark Brown 8 10 Control Dark Brown with oily Dark layer and Brown foul smell Turbid Brown and foul odour Turbid Brown and foul odour Turbid Brown and foul odour Turbid brown and foul odour Turbid Brown 50 8.7 50 8.7 70 8.7 60 8.7 115 8.6 65 8.8 65 8.8 55 8.8 60 8.8 6.08 6.12 6.10 6.17 6.17 6.24 6.11 6.15 6.12 6.11 1510 0.5 600 0.2 8.4 \ 13 2720 0.6 410 0.3 11.2 2570 0.1 1180 0.2 8.4 2860 0 435 0.2 7.2 2610 0 460 0.2 7 2630 0 460 0.2 7.2 2520 0 480 0.3 7.9 2520 0 415 0.2 8.1 2590 0 530 0.3 7.5 2410 0 400 0.2 7.9 2520 0 430 0.2 7.9 13.3 11.8 16.6 16.3 15 14.3 13.8 14.7 13.2 13 21.6 21.9 21.6 21.8 21.6 21.4 21.1 21.5 21.3 21.7 21.5 Note: All units in mg/L unless otherwise indicated. * UMR is under measuring range. I 1) Aerobic Treatment of Leachate (Control and Experiment) over a period of 20 days (continued). Colour Turbidity (NTU) PH Electrical conductivity (dS/m) Alkalinity ( mg/L as CaCO3 ) DO Ammonianitrogen Nitrite-nitrogen Nitrate-nitrogen Soluble reactive phosphorus o Temperature ( C) Dark Brown Experiment Light Light Brown Brown Dark Brown Light Brown Light Brown Light orange Light orange Clear orange Clear orange Clear Orange 110 8.2 70 8.6 25 8.7 15 8.2 15 8.7 10 8.6 5 8.4 5 8.3 5 8.2 5 8.7 5 8.8 6.64 6.38 6.27 6.14 6.04 6.00 5.91 5.78 5.72 5.72 5.78 1510 0.5 2630 6 2130 3 2670 3 2470 2 2210 2 1700 2 1350 3 980 2 990 4 910 4 600 0.2 8.4 450 0.2 8.7 1385 0.2 8.3 390 1.3 7.6 405 7.2 9.4 285 38 20 185 85 35 110 130 35 15 195 90 *UMR 200 87 UMR 205 100 13 11.6 12.2 17.6 17.2 15.2 14.6 13 13 12 11.3 21.6 22 21.8 22 21.9 21.4 20.9 21.8 21.6 21.9 21.9 II 2) Column Experiment Results. Initial Control Parameter/ Runs Colour Turbidity (NTU) pH Electrical Conductivity (µS/cm) Alkalinity ( mg/L as CaCO3) Dissolved Oxygen (DO) Ammonia-nitrogen Nitrite-nitrogen Nitrate-nitrogen Soluble reactive phosphorusExperiment Colour Turbidity (NTU) pH Electrical Conductivity (dS/m) Alkalinity (as mg/L as CaCO3) Dissolved Oxygen (DO) Ammonia-nitrogen Nitrite-nitrogen Nitrate-nitrogen Soluble reactive phosphorous Granular Activated Carbon (GAC) Transparent 0.29 6.8 1st Light Black 22 8.5 5th Clear transparent 10.7 9.3 Blast Furnace Slag (BFS) Deionised Water 10th 1st 5th 10th Clear transparent Clear clear Clear 2.89 12.3 0.85 1.58 9.19 11.75 11.83 11.82 Sand 1.48 143.4 418 532 3150 3560 3680 67.5 56.7 61.8 30 320 160 230 70 130 150 40 40 60 8.96 0.156 0.002 UMR 0.023 9.54 0.29 0.06 0.78 9.66 0.33 0.06 1.14 9.64 1.26 0.03 0.68 13.14 UMR 0.1 20.9 12.12 UMR 0.09 25.3 11.44 UMR 0.017 17.3 12.71 1.12 0.009 5.76 13.03 1.08 0.01 4.69 12.92 1.26 0.014 4.79 0.657 0.80 2.27 0.439 0.63 0.793 0.057 0.052 0.027 Dark Brown 58.8 8.38 89.5 Dark Brown 8.55 Dark Brown 121.5 8.69 Dark Brown 205.1 8.72 Leachate Dark Brown 315.5 8.9 Orange 52.75 9.33 Orange 45.9 9.53 Light Brown 51.05 8.705 Light Brown 45.1 8.775 Light Brown 42.55 8.795 10.48 10.47 10.22 10.15 10.795 10.155 9.865 9.840 9.960 10.015 4700 4420 4920 4600 3415 1915 1615 3990 4230 4360 2.19 975 0.20 9.46 7.76 1177.5 0.24 11.40 7.60 1115 0.28 7.60 7.70 1070 0.28 6.49 9.755 1090 0.156 12.3 9.205 1107.5 0.142 26.85 5.825 1137.5 0.121 20.8 9.305 1095 0.2095 13.35 10.205 1242.5 0.244 13.05 9.19 1160 0.223 12.8 25.3 8.95 9.41 7.94 6.235 2.42 1.5 17.9 14.2 15.25 1st Clear 1.72 7.37 Sand 5th Clear 2.39 7.44 10 Clear 2.29 7.54 th Note: All units in mg/L unless otherwise indicated. * UMR is under measuring range. III 3) Particle Size Distribution Analysis. Media GAC BFS Sieve Size (mm) 5 4 2.8 2.4 2 1 0.5 0.25 0.125 0.063 <0.063 5 4 2.8 2.4 2 1 0.5 0.25 0.125 0.063 <0.063 Mass retained on Sieve (g) 0 160 126 8 3 0 0 0 0 0 0 0 0 0 0 186.64 92.45 61.47 34.45 15.05 5.42 0.47 % of total mass retained on sieve 0 53.0 42.0 2.7 1.0 0.0 0.0 0.0 0.0 0.0 0.0 0 0 0 0 47.14 23.35 15.52 8.70 3.80 1.37 0.12 % of total mass passing sieve 100 47.00 5.00 2.30 1.30 0.00 0.00 0.00 0.00 0.00 0.00 100 100 100 100 52.86 29.51 13.99 5.29 1.49 0.12 0.00 IV 3) Media Sand Particle Size Distribution Analysis (continued). Sieve Size (mm) 5 4 2.8 2.4 2 1 0.5 0.25 0.125 0.063 <0.063 Mass retained on Sieve (g) 0 0 0 0 0.14 1.69 97.01 284.65 12.54 0.19 0.19 % of total mass retained on sieve 0 0 0 0 0.04 0.43 24.47 71.81 3.16 0.05 0.05 % of total mass passing sieve 100 100 100 100 99.96 99.54 75.07 3.26 0.10 0.05 0 V Appendix B 1) Temporal variations in Leachate Quality (1995-2009)-Figures 1 a-g represents leachate quality relative to time from SWMC landfill, Newcastle, Australia (1995-2009). Figure 1-a) Temporal variations in ammonia relative to time in leachate from SWMC landfill, Newcastle, Australia (1995-2009). Figure 1-b) Temporal variations in pH relative to time in leachate from SWMC landfill, Newcastle, Australia (1995-2009). VI Figure 1-c) Temporal variations in electrical conductivity relative to time in leachate from SWMC landfill, Newcastle, Australia (1995-2009). Figure 1-d) Temporal variations suspended solids relative to time in leachate from SWMC landfill, Newcastle, Australia (1995-2009). VII Figure 1-e) Temporal variations in TOC/BOD/COD relative to time in leachate from SWMC landfill, Newcastle, Australia (1995-2009). Figure 1-f) Temporal variations in Sodium and Potassium relative to time in leachate from SWMC landfill, Newcastle, Australia (1995-2009) VIII Figure 1-g) Temporal variations in chloride and sulphate relative to time in leachate from SWMC landfill, Newcastle, Australia (1995-2009) IX 2) Rainfall Data (1996-2009), Bhutan (Hydro-met Services Division, Department of Energy, MTI, Thimphu, Bhutan (1996-2009)). Station: 220m 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 70 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 25 0 15 0 0 17 24 11 0 3 5 0 0 6 8 86 2 0 0 0 10.3 0 24 23.4 2 0 39 0 0 0 2 0 0 0 0 0 0 0.2 0 0 0 19 10 0 0.2 0 0 12 0 0 0 0 0 0 0 30 11 0 0 15.5 22 94 22 29 0.5 0 6.1 3.5 6 57 0.2 0 11 0 74 41 0 2.1 20 6 0.6 6 19 9 1 4.5 24 19 2.5 7 2 0.5 71 29 20 20 50 42 25 27 32.7 110 4.3 2.8 2 3 6.5 0 13 0 0 7 0 15 0 1 0 0 44 3 0 79 66 8 15 29 86 29 100 90 7 62 26 46 32 18 7.6 18 2.2 0 95 9 7.5 0 70 100 21 25.2 6.2 39 34 30 44 2.5 8 0 5 23 0.7 0 47 0 8 10.2 14.6 2.2 0 0 12 10.2 2.5 0 0 0 0 2.5 28 39 10.5 2.5 0 14 0 0 0 0 0 22.5 0 0 0 0 0 0 0 22.9 120.8 0 0 22.5 25 0 0 0 0 0 0 10 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 5.6 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 4 0 0 0 0 0 0 0 0 0 5 4 0 Note: Rainfall in mm X 2) Rainfall Data (1996-2009), Bhutan (Hydro-met Services Division, Department of Energy, MTI, Thimphu, Bhutan (1996-2009)). (continued). Station: 375 m 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 32.8 0 0 0 0 11.8 0 0 0 0 0 0 0 0 0 0 0 17.6 0 0 0 0 0 16 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 6.2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.6 0 0 13.2 0 10.2 0 0 12.2 3.6 4.6 0 0 12.2 7.2 0 6.2 0 0 7.2 2.8 0 0 0 0 0 30.2 8.2 0 7.2 0 0 0 0 0 0 0 0 0 57.2 64.4 3.8 0 0 31.6 0.6 4.2 0 21 9 0 4.8 1.8 0 4.2 0 3.6 17.6 0 0 0 6.4 0 0 10.8 55.8 20 25 0 0 24.6 1.2 40.2 1 10.2 0 39 0 57.4 40.8 6.8 75.2 7.2 1.2 7 10.4 54.4 0 0 1 32.2 16.2 107.4 46.8 31.8 0 134.6 168.6 29 191.2 268.8 160 69 205.8 89 99.8 30.2 1.4 65.8 2.4 7.6 0 0 0 0 4.6 26.6 24 0 5.6 9.6 0 1.4 11.8 3.6 257.2 34.8 19.4 0 163 22.4 85.8 24.4 8 0 14.2 10.6 27 21 1.2 3.2 2 6.2 90.8 42.4 10.8 9.2 113 40.6 29.2 189 64 21.8 168.8 30.6 7.2 13 0 2.8 3.6 1.2 76.2 19.6 0 0 0 0 0 11.8 0 1.4 0 13.6 35.4 7 0 30.8 7.6 9 77.4 256.6 30.2 0 19 0 29.4 28.2 4.6 0 0 0 0 0 0 0 0 0 0 5.8 57.6 204.8 34.4 0 16.2 0 0 0 0 0 7.6 4.4 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.2 0 0 0 0 0 0 0 0 0 0 17.6 0 0 0 0 0 0 0 0 0 0 0 1 0 Note: Rainfall in mm. XI 2) Rainfall Data (1996-2009), Bhutan (Hydro-met Services Division, Department of Energy, MTI, Thimphu, Bhutan (1996-2009)). (continued). Jan Station: 1930m 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 9 0 10 0 11 0 12 0 13 0 14 0 15 0 16 0 17 0 18 0 19 0 20 0 21 0 22 0 23 0 24 0 25 0 26 0 27 0 28 0 29 0 30 0 31 Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.8 29.8 11.8 0 8.8 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.6 5.6 0 0 0 0 23.8 0 0 2 12.2 4.8 0 2.6 1.2 0 25.6 12 0 11.6 30.8 0 4.4 0 0 3.6 5.4 0 0 0 0 1.4 1.2 0 0 0 0 0 0 2.2 0 0 0 2 15.4 0 0 0 0 0 0 3.8 2.8 5.6 0 0 0 3.6 0 0 0 0 0 0 3 71.8 34 8.8 0 0.6 0 0 0 0 0 9.6 0 0.4 0 0 0 1 0 0 1.2 0 0 0 0 0 0 0 4 0 0 0 0 4.2 0 12.4 28.4 18.2 8.4 42.2 15.4 1.6 0 0 3.8 0 4.2 0 3.8 0 1.4 0.6 1.8 0 0 39.8 2.4 0 9.2 0 0 6.4 8 15.2 2 33.6 14 9.2 0.8 0 0 7.4 2.8 10.6 5.2 10.6 0 0 0 1.4 11.6 2.4 0.8 6.2 1.4 3 34.2 18 2 0.8 0.6 29.6 16.8 10 11.8 7.6 0.8 1 3.8 0.6 0 0 0 2.6 3.8 14.6 15.2 3.2 9.6 3.4 0 0 0 0 0 0 0 0.8 28 0 0 0 0 0 0 5.6 0 14.6 0 0 0 0 2.2 3.8 1 25.2 38.8 24.8 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.8 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.4 1.2 XII 2) Rainfall Data (1996-2009), Bhutan (Hydro-met Services Division, Department of Energy, MTI, Thimphu, Bhutan (1996-2009)).(continued). Jan Station: 2380m 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 9 0 10 0 11 0 12 0 13 0 14 0 15 0 16 0 17 0 18 0 19 0 20 0 21 0 22 0 23 0 24 0 25 0 26 0 27 0 28 0 29 0 30 0 31 Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 8.8 3 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.3 0 0 0 0.6 5.6 0 0 0 0 0 0 0 9.7 6.7 0 2.6 0 0 0 0 0 1.9 0 0 0 0 0 0 0 0 0 0 0 0.5 0 0 0 1.8 6.3 0.9 0 0 0 0 1.6 3.5 0.2 7.1 0.2 0 0 1.9 0 0 0 0 0 0 0 0 4.9 72.5 47.2 0 2.3 0 0.7 0 1.1 9 0.4 15 1.4 3.1 0 0.6 0 0 0 0 0.8 0 0.3 0 0 1 0.8 0 0 0 0 0 0 0 0 0 4.8 0 2.8 2.9 0.9 0 0 10.6 0.6 0.2 0.6 2.6 0.6 0 0 0 0 0 3.2 1.9 0 1.9 0 1.5 0 2.2 14 1.4 3.3 13.4 0 3.2 0 0 0 20.5 18.7 26.4 9.2 0.6 0 12 6.2 13 0 0 2.8 0 0.5 8.1 3.7 0 0 15.6 8.7 4.2 24.4 1.7 0.1 1.4 7.1 0.2 1 1 6.9 3.4 0 0 6.3 1.4 3.1 7.1 0 4.6 0 1.2 0 0 0 0 0.2 0.1 1.8 0 0 0 0 1.4 1.6 0.3 0 0 0 0 0 0 0 0.3 27.7 81.4 0.1 0 0.1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Note: Rainfall in mm XIII 2) Rainfall Data (1996-2009), Bhutan (Hydro-met Services Division, Department of Energy, MTI, Thimphu, Bhutan (1996-2009)). (continued). Station:2760m 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 3 3.1 4 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 0 0 0 0 0 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 3 3 2 4 2 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 10 0 0 6 0 0 5 15 5 0 0 13 0 0 13 27 0 27 0 0 0 4 1 2 6 11 8 2 4 14 1 1 0 0 0 2 0 0 2 3 0 2 3 0 3 0 2 0 2 4 3 0 0 3 2 0 0 3 3 4 3 3 3 5 18 5 3 5 4 0 2 3 0 3 1 1.2 2.4 10 1 4 3 1 0 17.2 5.2 1 1.4 1.2 12 14 31.4 6.4 2 8 18 5 8 17.4 12.4 13.4 17 6.4 14 9 9.4 3.4 26.4 6.4 11 6 21.4 13 11.6 4 1.2 9.4 14.4 16.4 43 6 1 0 30.4 23 7.4 0 9.4 1.5 10 13 22 24 2.4 10.4 28.8 12.4 12 18 20.4 12.4 40 17.4 1 1.4 11 4 7.4 24 22 7.4 13.4 7 11 7.4 4.4 14.4 2.2 3.4 6 0 23.2 23.2 14.4 20 26.4 11.4 3.2 17 10.4 4 14.2 0 2 2 4 0 4.4 12 35.4 2.4 8 8.4 0 1 2 2.4 0 0 0 0 0 0 2 2 0 0 15 0 4 0 7.2 9 0 2.2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.2 4.3 0 0 0 0 0 0 0 0 0 0 0 - Note: Rainfall in mm XIV 3) Monthly Rainfall Data (1995-2009), Williamtown Meteorological Station (site number 061078), Newcastle, Australia. Year Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Annual 1995 105.4 54.4 194.8 11.6 188.4 101.6 1.8 0 115.2 30 146.8 87.8 1037.8 1996 117.8 94.6 78.6 4.6 112 156.6 47.4 73.4 62 48 87 60.4 942.4 1997 99.2 152.2 101.8 42.2 135.4 128.2 190.4 40.8 94.6 38.8 11.2 44.4 1079.2 1998 67 95.6 27.6 104.8 340 156.8 102.8 169 94.4 69.2 241.4 91.6 1560.2 1999 72.2 252 46.6 332.2 27.6 212 141.8 96.2 58.8 129.2 111.8 61 1541.4 2000 60.8 35.4 359.8 104.6 59.2 91.6 43.8 66.8 25.8 35.8 79 33 995.6 2001 62.2 113.2 154.2 103.8 410.2 14.6 74.2 41.2 54 38 105.4 56 1227 2002 17.2 270.4 126 68 129 82.8 36.6 61.2 45.2 7 38.4 171.8 1053.6 2003 9.6 74.6 58 167.8 178.8 29.8 94.6 50 0.4 74.4 115 44.8 897.8 2004 140.8 178.6 90.8 56.6 20.6 25.6 78.6 103.6 91.2 186.4 74.6 68.4 1115.8 2005 68.4 143 220.8 34.6 222.4 88.4 16.6 0.8 49.8 55.8 82 14.4 997 2006 41.2 99.8 78 90.4 79.4 105 101.8 100.2 162.2 12.8 126.8 68.6 1066.2 2007 14.8 59.2 137.6 250.4 42.6 414.2 28 92.8 50.8 49.4 104 85.2 1329 2008 181.4 222.6 64 256.6 35.8 142.6 92.8 34 179.2 76.2 88.2 90.2 1463.6 2009 20.6 229.8 49.2 196.2 153 112.6 50.2 1 20.2 61.4 Note: Rainfall in mm. XV 4) Monthly Temperature (mean maximum) Data (1995-2009), Williamtown Meteorological Station (site number 061078), Newcastle, Australia. Year Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Annual 1995 26.3 26.9 25.8 23.2 20.6 17.5 17.2 21.9 19.9 24.2 26.3 25.1 22.9 1996 26.7 25.9 25.2 24.2 21.2 18.8 17.2 19.3 22.4 23.4 25.2 26.6 23 1997 26.2 27.6 26.4 24.8 20.5 18.2 16.6 19.1 20.1 24.6 28.1 30.5 23.6 1998 30.2 30.4 28.3 24.7 20.5 17.2 16.4 18.6 22.1 23.7 22.9 26.8 23.5 1999 28.8 26.9 27.2 21.9 21.6 17.7 17.5 18.9 21.8 24.3 23.3 25.8 23 2000 26.3 29.6 25.9 23.5 20 17.2 17.8 18.4 23.4 24.2 24.6 29.7 23.4 2001 30.9 29.4 26 24.3 19.2 19 17.6 19.3 22.3 24.5 24.6 28.2 23.8 2002 29 26.7 26.5 24.3 20.2 18 18.4 19.8 22.8 27 28.3 27.8 24.1 2003 29.5 28.2 25.1 22.5 20.5 19.3 17.3 18.9 23.3 22.5 25.2 28 23.4 2004 30 29.5 26.3 24.2 20.7 19 17.6 19.5 21.8 23.7 26.9 26.6 23.8 2005 28.6 28.2 25.2 24.9 20.4 18.5 18.3 20 21.2 25 26.2 30.7 23.9 2006 29.6 29.9 27.3 24.4 20.1 17.3 17.6 19.5 23.2 25.1 26.5 26.1 23.9 2007 29.5 28.6 27.6 23.2 22.3 16.7 17 20.3 21.9 26.9 25.1 26.2 23.8 2008 27.5 25.3 25.8 21.9 20.5 18.5 17.2 17.6 22.1 24.4 24.7 27.9 22.8 2009 30.2 28.4 27 23.3 20.6 18.1 17.7 21.6 23.7 23.1 28.5 27.5 24.1 o Note: Temperature in C. XVI 5) Leachate Monitoring Data (1995-2009) from SWMC, Newcastle, NSW, Australia. Date 5.07.1995 31.07.1995 3.08.1995 10.08.1995 17.08.1995 25.08.1995 7.09.1995 14.09.1995 21.09.1995 28.09.1995 5.10.1995 13.10.1995 20.10.1995 27.10.1995 3.11.1995 10.11.1995 16.11.1995 20.11.1995 21.11.1995 22.11.1995 23.11.1995 24.11.1995 1.12.1995 31.01.1996 15.02.1996 8.03.1996 12.04.1996 10.05.1996 12.06.1996 26.07.1996 23.08.1996 26.09.1996 24.10.1996 1.11.1996 21.11.1996 20.12.1996 Ammonia 0.05 0.01 0.01 0.01 0.01 0.01 0.01 0.01 6.07 29.5 9.62 9.37 8.62 7.66 5.53 4.03 3.69 5.15 3.99 2.5 10.2 10.7 16.6 15.8 12.01 14.1 20.4 37.3 25.5 35.5 30.1 49.7 52.6 41.4 33.2 Nitrite Nitrate EC (us/cm) pH Alkalinity (mg/L of CaCO3 BOD COD Fl Cl Sulphate Ca Mg K Na 0.76 161 103 140 133 156 27 51 80 232 126 138 179 150 170 172 160 390 280 150 98 110 60 59 66 54 52 5 5 133 103 112 117 124 88 131 280 122 139 111 110 117 93 117 175 105 117 8 9 6 7 19 63 27 29 145 155 48 69 100 209 116 128 171 147 131 110 107 1 31 70 75 73 78 53 146 119 99 83 97 96 138 110 90 75 86 90 33 27 22 25 25 26 157 132 109 96 102 106 104 92 60 183 89 57 190 101 71 264 Fe Pb 5 0.01 0.12 0.01 0.01 0.06 0.01 0.02 0.01 0.01 0.02 0.01 0.01 0.01 0.01 270 336 623 723 730 522 460 569 714 686 632 840 676 580 418 528 478 962 0.01 2368 0.01 0.08 2219 2360 2500 7.8 7.2 7.3 7.3 6.4 6.6 6.6 8.1 7.9 8 7.9 7.2 7.8 7.7 8 6.5 7.5 0.4 0.2 2710 2660 7.5 7.5 0.01 0.04 0.07 0.03 0.03 0.03 0.03 7.7 7.6 7.8 7.3 6.8 6.6 7.6 6.6 6.3 7.3 7.4 1850 0.04 2250 800 820 3 0.65 31 57 42 694 2750 857 1060 1060 1170 1060 1160 1070 1080 983 663 1080 1260 1860 783 265 70 40 351 101 41 25 482 271 26 365 102 81 985 3850 1280 1420 1540 1160 1410 1380 1130 1150 1070 818 1510 2270 1260 545 231 171 609 205 242 174 855 465 274 174 0.46 0.22 0.82 0.22 0.11 0.51 0.57 0.54 2.7 2.1 0 0 0 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0 0.1 0.1 0.1 0.1 0.1 0.1 6 0.72 280 95 0.49 235 28.6 0.59 318 39 86 426 0.1 0.1 16 0.1 XVII Date 31.01.1997 20.02.1997 20.03.1997 16.04.1997 12.05.1997 14.05.1997 16.05.1997 4.06.1997 17.07.1997 14.08.1997 25.09.1997 31.10.1997 19.11.1997 20.11.1997 18.12.1997 21.01.1998 19.02.1998 26.03.1998 23.04.1998 5.05.1998 7.05.1998 30.06.1998 23.07.1998 27.08.1998 30.09.1998 22.10.1998 30.11.1998 24.12.1998 21.01.1999 28.01.1999 4.02.1999 9.02.1999 24.03.1999 22.04.1999 24.05.1999 1.07.1999 21.07.1999 11.08.1999 Ammonia 11.4 53.5 57.3 25.7 6.99 16.2 17.4 15.2 86.7 Nitrite Nitrate 0.01 0.12 0.43 0.03 23.2 57.3 42 9.73 28.3 63.5 35.7 62.9 95.9 158 164 145 167 266 84.5 46.2 74.9 35.9 22.4 17.8 37.2 44.7 64.4 105 51.5 96.3 202 0.46 0.01 0.02 0.07 0.01 0.01 0.02 0.03 EC (us/cm) 2612 1378 1261 1051 1300 2045 3934 3840 1613 3590 3760 3500 4360 4840 1860 4476 3860 3222 3685 4567 0.01 0.04 0.03 0.01 0.01 2720 3277 3366 4137 3360 1653 1731 2.09 1883 2250 4137 0.08 3120 5410 pH 7.3 7.3 7.4 8.1 6.4 6.8 6.8 7.2 7.1 7.6 7.2 7.8 8.3 7.3 8.9 8.7 8 7.7 7.8 6.9 6.9 7.3 7.7 7.6 7.3 7.7 8.7 8.6 7.2 7.4 8.3 6.8 7.4 7 7.7 6.8 7.13 7.6 Alkalinity (mg/L of CaCO3 998 190 329 1470 1560 972 1200 2180 1160 76.2 675 877 1610 2560 BOD COD 26 99 285 22 239 361 395 421 1560 530 140 164 57 404 322 474 167 26 47 68 23 96 781 930 69 48 62 74 17 14 40 17 28 89 107 19 23 92 500 828 533 375 314 495 404 454 1080 1510 414 438 424 534 149 344 531 293 128 243 320 168 230 789 451 1440 1110 Fl Cl Sulphate Ca Mg K Na 0.55 279 47 82 94 76 254 0.1 0.46 0.54 82 118 48 49 26 29 12 86 0.1 0.1 408 1 84 145 98 352 0.1 1.3 681 13 8.4 211 621 0.1 0.82 650 5 36 192 549 705 2310 973 Fe Pb 564 372 528 1.7 0.1 262 314 20 27 0.1 0.1 0.64 0.42 333 353 34 1 116 1.6 94 108 # 83 99 0.8 537 14 221 155 ## 474 0.1 1.08 537 38 34 123 ## 421 0.1 0.32 0.36 0.36 204 211 202 121 77 39 29 48 65 53 57 60 49 38 51 198 203 196 0.1 0.1 0.1 0.91 547 35 89 138 ## 434 0.1 0.54 580 1 154 166 ## 530 26 XVIII Date 6.09.1999 7.10.1999 10.11.1999 2.12.1999 13.01.2000 9.02.2000 6.03.2000 5.04.2000 25.05.2000 15.06.2000 12.07.2000 10.08.2000 24.08.2000 7.09.2000 11.10.2000 7.11.2000 5.12.2000 21.12.2000 12.01.2001 7.02.2001 7.03.2001 11.04.2001 10.05.2001 5.07.2001 16.08.2001 6.09.2001 14.11.2001 11.12.2001 7.01.2002 6.02.2002 21.03.2002 24.05.2002 20.06.2002 7.08.2002 11.09.2002 2.10.2002 22.10.2002 22.11.2002 Ammonia Nitrite 165 88.6 66.5 137 136 110 200 136 227 223 223 268 430 173 168 192 128 62.8 68.4 79.3 73.6 483 206 874 970 146 180 94.3 970 685 479 564 514 842 458 429 Nitrate 0.1 5090 4910 3520 9200 7910 6490 10800 3690 8390 6910 7590 7010 0.01 1200 9010 9200 0.04 0.16 0.09 0.03 EC (us/cm) 0.02 0.01 0.01 0.01 0.01 0.01 0.04 0.01 0.05 0.01 6290 8740 3360 3320 2320 2330 11400 9600 16300 16400 7250 9800 2450 16500 12100 9870 10900 11100 1530 13000 pH 7.56 7.15 7.8 8.12 7.44 7.53 7.66 6.93 7.54 8.26 8.42 8.46 8.1 7.91 8.59 8.64 7.3 8.17 8.23 6.94 7.89 6.55 6.9 7.71 8.2 7.51 7.24 8.76 8.69 7.27 7.37 8.07 7.93 7.61 7.91 7.93 8.49 8.73 Alkalinity (mg/L of CaCO3 1320 1960 3370 3350 2730 BOD COD 249 121 175 55 151 22 92 191 308 430 460 644 117 378 208 148 134 368 352 664 610 461 429 625 297 927 388 784 588 744 952 2080 734 739 1140 1010 614 1380 444 390 940 748 795 3720 6950 771 4710 3940 4230 4370 204 524 162 181 143 266 213 179 113 93 504 435 582 80 75 37 562 1032 211 964 1610 1550 1320 798 1010 667 1730 1340 1430 1160 1430 2020 1830 2660 Fl Cl Sulphate Ca Mg K Na Fe 0.53 388 8 97 93 ## 466 4.1 0.6 ### 20 60 211 ## 748 9.6 1.1 ### 96 122 241 ## 855 99 0.9 ### 79 25 76 216 ## 910 12 1.2 ### 106 16 55 250 ## ### 9.6 0.5 463 65 107 90 ## 312 15 0.4 0.3 220 44 10 63 68 59 50 86 77 178 166 8.5 8.1 1 ### 90 87 199 ## ### 20 1.2 ### 55 117 225 ## ### 20 0.4 262 81 44 40 92 196 4.7 1.1 ### 95 101 190 ## ### 12 1.2 ### 45 99 184 ## ### 26 1.2 1.4 ### ### 86 111 103 78 196 201 ## ## ### ### 8.9 12 Pb 0 0 XIX Date 23.01.2003 5.02.2003 3.03.2003 7.04.2003 21.05.2003 3.06.2003 15.07.2003 18.08.2003 1.09.2003 2.10.2003 30.10.2003 1.12.2003 6.01.2004 18.02.2004 2.03.2004 6.04.2004 6.05.2004 21.06.2004 1.07.2004 5.08.2004 1.09.2004 30.09.2004 3.11.2004 1.12.2004 11.01.2005 1.02.2005 2.03.2005 12.04.2005 24.05.2005 2.06.2005 4.07.2005 3.08.2005 1.09.2005 5.10.2005 14.11.2005 5.12.2005 10.01.2006 1.02.2006 Ammonia 423 880 183 502 241 264 486 197 241 262 356 74.2 256 83.4 108 122 334 646 490 445 342 170 128 156 193 100 197 240 330 275 46 328 296 311 553 92.9 26.8 122 Nitrite Nitrate 0.06 0.61 0.03 0.08 0.01 0.36 0.01 0.06 0.04 0.01 0.203 0.458 0.077 0.013 0.067 0.018 0.045 0.01 EC (us/cm) pH 11600 15300 8030 9500 5660 6270 9640 7380 8300 11300 10200 4650 7950 7900 4920 4750 9170 11200 11200 10100 6520 5170 5220 6900 7090 7310 7600 6960 5290 6050 1610 5150 8190 9100 9870 3510 1550 7930 7.98 8.18 8.16 8.26 8.06 8.09 8.13 8.8 8.9 8.79 8.45 8.9 8.41 8.63 8.61 8.62 8.71 8.09 8.35 8 8.58 8.26 8.36 8.4 8.49 8.63 8.32 8.53 7.88 8.05 8.37 8.43 8.76 8.5 7.97 7.48 7.24 8.35 Alkalinity (mg/L of CaCO3 5550 1460 2050 1150 2070 2750 2200 1140 1780 2140 BOD COD 92 66 582 318 164 6 14 298 76 20 124 111 165 112 40 88 44 63 206 143 51 59 107 96 96 135 53 59 10 50 31 17 252 822 171 24 2 356 1730 664 1510 855 363 574 887 1400 936 1310 1420 646 1370 891 431 555 921 1230 1040 1200 896 607 730 1190 1440 1310 890 1000 441 358 79 466 1980 1680 1620 403 166 1800 Fl Cl Sulphate Ca Mg K Na Fe 1.3 ### 76 53 222 ## ### 16 0.8 649 369 160 83 ## 432 2 1.3 ### 125 62 129 ## 862 8.4 0.6 908 132 26 79 ## 655 1.3 1.2 ### 85 29 150 ## ### 1.9 1 ### 140 40 146 ## ### 4.6 1 ### 103 54 108 ## 811 4.7 0.6 810 125 34 57 ## 623 4 1 ### 108 45 110 ## ### 2.8 0.6 768 121 61 54 ## 429 3.7 0.8 844 164 94 78 ## 607 9.3 1.5 ### 126 68 128 ## ### 12 1.5 146 137 63 116 ## ### 8.4 Pb 0 0 0 XX Date 1.03.2006 11.04.2006 3.05.2006 31.05.2006 3.07.2006 2.08.2006 5.09.2006 11.09.2006 15.09.2006 3.10.2006 1.11.2006 4.12.2006 22.01.2007 5.02.2007 5.03.2007 2.04.2007 2.05.2007 1.06.2007 13.06.2007 22.06.2007 29.06.2007 5.07.2007 2.08.2007 11.09.2007 2.10.2007 7.11.2007 3.12.2007 8.01.2008 11.02.2008 26.03.2008 2.04.2008 7.05.2008 11.06.2008 3.07.2008 5.08.2008 4.09.2008 1.10.2008 17.11.2008 Ammonia 89.3 402 502 218 420 193 535 42 53.8 590 437 488 363 251 108 252 191 252 49.9 86.5 150 123 72.4 255 437 294 399 471 154 353 468 308 209 105 91.6 148 85.8 248 Nitrite Nitrate 0.083 0.048 0.051 0.01 0.01 0.015 0.011 0.01 0.039 0.386 0.104 0.027 0.013 0.019 0.01 0.01 0.01 0.012 0.01 0.01 0.01 0.044 0.073 0.01 0.021 0.149 0.039 0.038 0.555 0.02 0.06 EC (us/cm) pH 2300 9710 5780 6210 7170 4570 10800 1600 1900 10500 15200 11200 11200 15900 3500 7780 3490 5130 2120 2800 3990 3480 2930 6530 8490 10600 8190 11900 3780 7900 10100 7270 4700 3310 2980 5890 2720 5450 7.35 8.17 8.55 8.39 8.14 8.22 7.16 6.01 6.31 7.78 8.02 7.93 7.72 7.85 7.13 7.32 7.23 7.64 6.84 6.68 7.14 7.27 7.78 7.72 7.42 8.14 7.63 7.6 7.23 7.98 7.88 7.97 7.97 7.58 7.58 8.07 7.9 7.76 Alkalinity (mg/L of CaCO3 BOD COD 72 112 89 242 286 395 343 438 398 306 215 129 89 256 21 103 1230 301 1320 1200 721 949 43 984 118 218 468 170 120 106 70 65 41 28 45 40 41 46 270 1830 1800 1350 1730 1380 1600 850 840 1940 1810 1230 1660 1710 522 768 2030 1260 1990 2080 285 1880 1280 1600 1490 1760 495 1070 1040 611 376 230 175 428 310 573 Fl Cl Sulphate Ca Mg K Na Fe Pb 1.5 ### 86 64 111 ## ### 7.9 0 0.7 773 84 97 80 ## 641 4.9 0.3 0.5 192 248 81 104 52 86 29 36 43 64 161 240 7.1 12 1.1 ### 92 126 202 ## ### 9.9 1.4 ### 88 95 223 ## ### 12 0.5 375 53 81 73 ## 394 16 0.4 0.5 268 389 40 45 104 106 69 78 84 ## 257 401 22 16 0.5 363 164 65 46 56 482 5.5 1.1 ### 73 109 183 ## ### 6.5 0.6 583 20 81 65 ## 418 11 0.7 890 48 79 105 ## 727 9.9 0.6 402 196 62 59 71 422 20 0.7 763 74 51 74 ## 598 8.3 0 0 XXI Date 1.12.2008 19.01.2009 4.02.2009 30.03.2009 28.04.2009 12.05.2009 Ammonia 275 375 418 245 479 735 Nitrite Nitrate 0.5 0.5 0.1 0.1 EC (us/cm) pH 5140 9790 10800 9560 8760 9880 7.72 8.28 8.49 8.56 7.97 8.06 Alkalinity (mg/L of CaCO3 BOD COD 46 649 96 116 52 71 443 1650 1580 1330 746 1010 Fl Cl Sulphate Ca Mg K Na Fe 1.2 ### 10 119 185 ## ### 8.5 1.1 ### 71 114 162 ## 970 7.2 Pb 0 Note: All units in mg/L unless otherwise indicated. Blank cells indicate “No Sample”. XXII