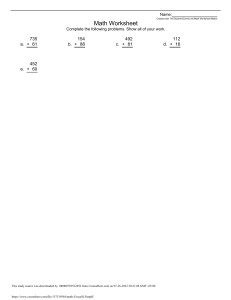
Tutorial 3 Protein Lab: Simulation of protein purification Section Tuesday afternoon This study source was downloaded by 100000773676871 from CourseHero.com on 11-10-2022 18:25:48 GMT -06:00 https://www.coursehero.com/file/58423324/chem-271-tutorial-3docx/ Protein #1 I had to isolate protein #1, its enzyme activity is stable for several hours at a temperature 40 °C. The enzyme activity of protein had pH values of 2.5 and 9.5 Table 1 Default mixture Method protein Initial 511 Enzyme 4000 Yield 100% Cost 0 Theory I began purifying my protein by the method of ion exchange chromatography. Ion exchange chromatography; is when the polymer resin is negatively charged or positively charged, it allows for a separation, as if we have an anionic resin, it will attract positively charged ions that are loaded into the ion exchange 1 Agbooth This study source was downloaded by 100000773676871 from CourseHero.com on 11-10-2022 18:25:48 GMT -06:00 https://www.coursehero.com/file/58423324/chem-271-tutorial-3docx/ chromatography2. The higher the pH the less protonated the protein will become, basically it separates proteins by their charge The ion exchange chromatography was done at a fraction range of 10-35. Secondly, to purify my protein further, I conducted a gel filtration. I started off with my protein at a pH of protein 1 above 8, with a Mz of about 50k. I started off by doing an ion exchange chromatography. This ion chromatography took place in Q-Sepharose with a pH gradient of 2.5-7.9. This ion exchange was done with a buffer of pH 7. Results Table 2. Results of protein 1 Total Protein Total enzyme Enrichment Yield Cost(hours/10 0 units) 43.mg 3999 Units 11.9 100% 0.175 Discussion I preceded by the means of ion chromatography. This was done in a ion exchange media of Q- sepharose at a fraction of 10-30. This allowed for the protein to be positive and to isolate my protein at pH of 8.1, in one step. It was successful as it I managed to keep the full yield of product, without any lose. I also received a protein enrichment of 11.9, which is a pretty high value. Enrichment; 3 is a technique in which allows for concentration of proteins to improve within analysis. This method of separation is also verily cheap as it only costs 0.175, in which the cost comes out to hours/100 units. Additonally, I only lost 1 unit of enzyme, which is slim to none . Q-sepharose was used, as its matrix type is a strong 4anion exchanger, unlike ssepharose that is a cation exchanger. The purpose of using this media is that it causes for the protein to be positive. I also in the end did a gel filtration to see if there was any effect, but it stayed the same 2 Chemlibre 3 4 J. Powlowski This study source was downloaded by 100000773676871 from CourseHero.com on 11-10-2022 18:25:48 GMT -06:00 https://www.coursehero.com/file/58423324/chem-271-tutorial-3docx/ I tried using many other separation methods, however they always caused for an immediate loss in yield. When trying to do use Ammonium sulphate, my yield dropped to 41.3%, which isn’t good as were supposed to keep a yield of over 90%. Heat treatment did not work either; there was no loss in yield, however this separation method did not purify my protein at all, it was if I didn’t apply any separation method at all. Protein #4 Theory I once gain started with the method of ion exchange chromatography. As explained above once again, ion exchange chromatography; is when the polymer resin is This study source was downloaded by 100000773676871 from CourseHero.com on 11-10-2022 18:25:48 GMT -06:00 https://www.coursehero.com/file/58423324/chem-271-tutorial-3docx/ negatively charged or positively charged, it allows for a separation, as if we have an anionic resin, it will attract positively charged ions that are loaded into the ion exchange chromatography. Where the whole goal is to separate proteins by charge. Secondly I did hydrophobicity5; the purpose of this separation technic is that it separates proteins from one another by hydrophobicity. A salt can be used to decrease the amount hydrophobicity among proteins. Gel filtration6; the sample is loaded into the gel filtrate, where it has porous polymer beads; typically made of carbohydrates. This separation method is driven by the size of the protein, where small proteins will be caught in the polymer beads, while large proteins will come out of the gel filtration, as they cannot be caught in the polymer. Lastly, Ammonium 7 sulphate fractionation was done. This is when proteins are separated by their solubility, when a salt is present. Table 3. Initial data for protein 4 Inital 511 Eznyme 7000 yield 100% Enrichment 1.0 cost Results Table 4. Results for protein 4 Initial Ion Exchange Ammonium Protein (mg) 330 239 Enzyme 7000 6997.6 Yield 100 100 Cost 0.100 0.129 sulfate Hydrophobic 207 6980.5 99.7 .229 interaction Gel filtration 101.9 69.59.1 99.4 .704 Discussion 5 Chem Libre 6 Lehman 7 Chem Libre This study source was downloaded by 100000773676871 from CourseHero.com on 11-10-2022 18:25:48 GMT -06:00 https://www.coursehero.com/file/58423324/chem-271-tutorial-3docx/ The enzyme of of protein 4 can be heated for several hours at a temperature of 40 °C. It has pH values between 4.5 and 10. It has a Mr of about 40K, at about a pH of 6.1. To start off I had a very difficult time trying to purify my protein, I did this for about five hours and tried many combinations of separation to try to get it down to one protein but it just seems not to work out for me. I got down to 4 proteins including my protein. I started off the experimental procedure by putting my protein solution through ion chromatography in a Q-sepharose media using a salt gradient from 01.5, I fractioned this at 40-54. I then proceeded to do an ammonium sulphate fractionation. This salting out affects the solubility, of large groups of proteins therefore purifying further. I then followed this step with a hydrophobic interaction Chromatography to separate them by their hydrophobicity. I did this in a PhenylSepharose CL-4B media that was fractioned from 45-69. The last step was Gel filtration with a polyacrylamide/agarose gel type; specifically Ultro gel AcA 34b, fractioning between 46-59. This purified my protein as much I could. The Enrichment increased the more separation methods,that were done. I ended with 101.9g of protein as I started with 511 mg. I ended with a cost of 0.704 for all the separation methods This study source was downloaded by 100000773676871 from CourseHero.com on 11-10-2022 18:25:48 GMT -06:00 https://www.coursehero.com/file/58423324/chem-271-tutorial-3docx/ Reference J.Powlowski, M.J. Kornblatt, P.Joyce, J. Turnbull and Mihai Ciortea Chemistry 271 Laboratory and Turorial Manual edition 1.15 Concordia , 2019 John W. Lehman Operational Organic Chemistry: A Problem Solving Approach to the Laboratory Course 4th Edition, Pearson Prentice Hall, Upper Saddle River,NJ Lisa Nichols. Libre Texts: Organic Chemistry Laboratory Techniques, Butte Community College, 2019 This study source was downloaded by 100000773676871 from CourseHero.com on 11-10-2022 18:25:48 GMT -06:00 https://www.coursehero.com/file/58423324/chem-271-tutorial-3docx/ Powered by TCPDF (www.tcpdf.org)