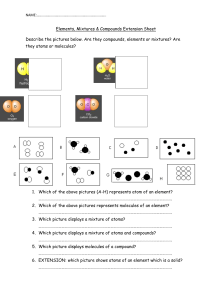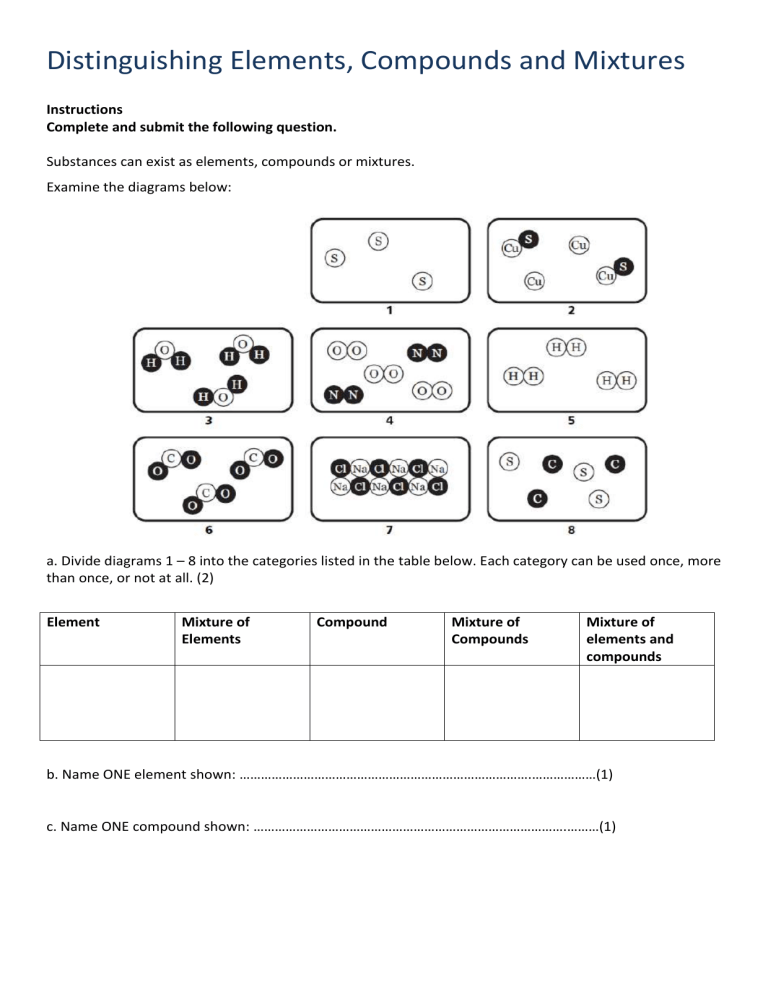
Distinguishing Elements, Compounds and Mixtures Instructions Complete and submit the following question. Substances can exist as elements, compounds or mixtures. Examine the diagrams below: a. Divide diagrams 1 – 8 into the categories listed in the table below. Each category can be used once, more than once, or not at all. (2) Element Mixture of Elements Compound Mixture of Compounds Mixture of elements and compounds b. Name ONE element shown: ……………………………………………………………………….………………(1) c. Name ONE compound shown: …………………………………………………………………………….………(1) Essential Vocabulary Purpose: The use of this language in your work when responding to questions and constructing texts demonstrates your level of understanding of the content and skills of this topic. Use the highlighter tool and the key provided to show: Words I know and can use correctly in a sentence about the topic Words I know or have heard of but could use not use them correctly in a sentence about the topic Words I don’t know yet Word / Term Atom Molecule Element Compound Symbol Formula Chemical change Law of conservation of mass Law of conservation of energy Collision Theory Chemical reaction Reactants Products Acid Base Salt Carbonate Notes Corrosion Decomposition Neutralisation Combustion Precipitation Precipitate Temperature Exothermic Endothermic pH Indicator Society Elements, Compounds and Mixtures worksheet 1. Which of the boxes contains: a) Molecules of an element? b) Molecules of a compound? c) Atoms of an element? d) Do any of the boxes contain a mixture? Explain your answer. 2. Look at the following substances. Circle the elements and underline the compounds a) Li b) N2 c) CO d)Cl2 e)S8 f)NaCl 3. How many elements make up each compound? a) HCl b) CaCO3 c) HNO3 d) H2SO4 e) CH3OH 4. How many atoms are there in each of these molecules? a) N2 b) CO2 c) O3 d) CH4 e) SO2 f) C2H4 5. Circle the molecule with the most atoms, in each pair; a) HCl or POCl b) NH3 or HNO3 c) C3H8 or C2H5OH d) CH3COCH3 or C6H6 6. What is the formula for each molecules shown below O O O C O O H H H N H H