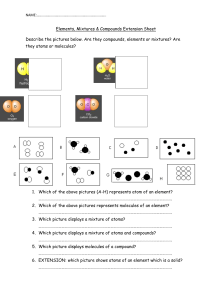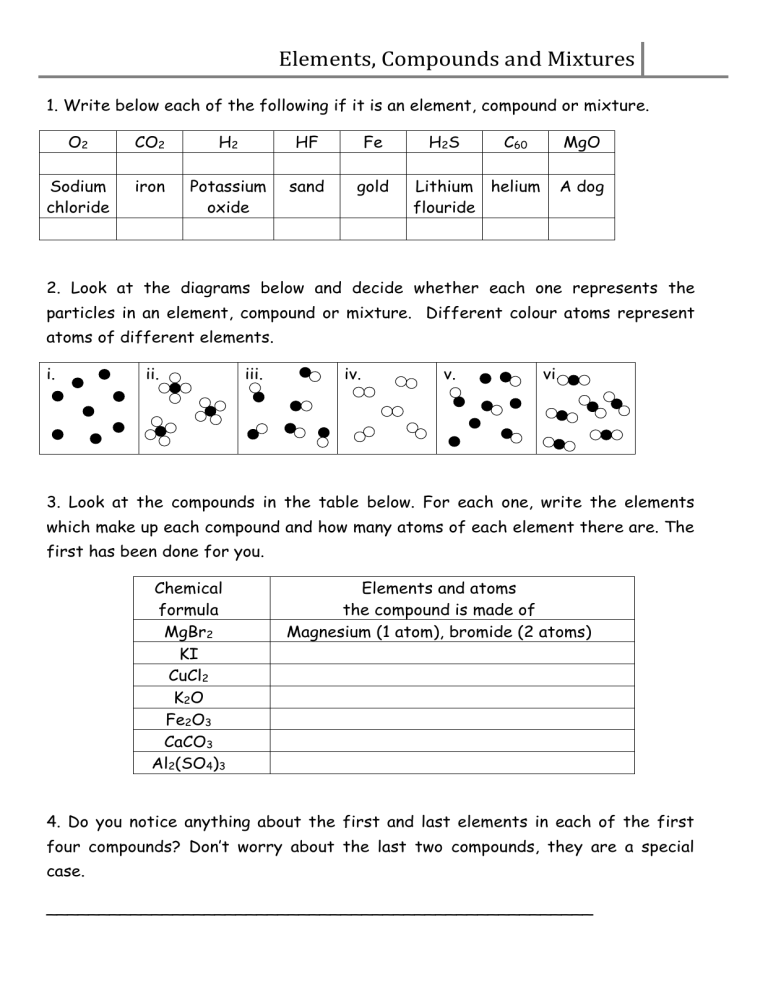
Elements, Compounds and Mixtures 1. Write below each of the following if it is an element, compound or mixture. O2 CO2 H2 HF Fe Sodium chloride iron Potassium oxide sand gold H2S C60 Lithium helium flouride MgO A dog 2. Look at the diagrams below and decide whether each one represents the particles in an element, compound or mixture. Different colour atoms represent atoms of different elements. i. ii. iii. iv. v. vi. 3. Look at the compounds in the table below. For each one, write the elements which make up each compound and how many atoms of each element there are. The first has been done for you. Chemical formula MgBr2 KI CuCl2 K2O Fe2O3 CaCO3 Al2(SO4)3 Elements and atoms the compound is made of Magnesium (1 atom), bromide (2 atoms) 4. Do you notice anything about the first and last elements in each of the first four compounds? Don’t worry about the last two compounds, they are a special case. ____________________________________________________