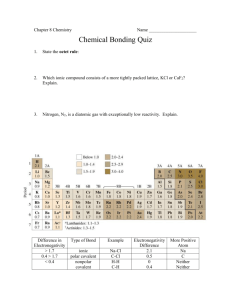
Name: _____________________________________________________________________________ Period: ____ Date: ________________________ Unit 7: Bonding Test Review Sheet Periodic Trends: 1. Define: a) Ionization energy b) Electronegativity 2. Atomic radius INCREASES or DECREASES as you go down a group, because: Atomic radius INCREASES or DECREASES as you go left to right across a period, because: Electronegativity INCREASES or DECREASES as you go down a group, because: Electronegativity INCREASES or DECREASES as you go left to right across a period, because: Ionization energy INCREASES or DECREASES as you go down a group, because: Ionization energy INCREASES or DECREASES as you go left to right across a period, because: 3. On the periodic table below, draw labeled arrows to show the direction of increasing atomic radius, electronegativity, and ionization energy. 4. Group 1 elements GAIN or LOSE ____ electron(s) to form an ion with a _______ charge. Group 2 elements GAIN or LOSE ____ electron(s) to form an ion with a _______ charge. Group 6 elements GAIN or LOSE ____ electron(s) to form an ion with a _______ charge. Group 7 elements GAIN or LOSE ____ electron(s) to form an ion with a _______ charge. Shorthand Electron Configuration: 5. Write the shorthand electron configuration for the following: Mg ________________________________________________________________ Fe ________________________________________________________________ Br- ________________________________________________________________ Al3+________________________________________________________________ Types of Bonds: 6. Ionic bonds: a) Electrons are _________________________________________, and atoms in the bond are attracted to each other because b) Are formed between a ___________________________ and a _______________________________. c) Are formed between two atoms that have a electronegativity difference of close to 0, between 0.4-2, or >2 7. Non-polar covalent bonds: a) Electrons are ______________________________________________________. b) Are formed between a ___________________________ and a _______________________________. c) Are formed between two atoms that have a electronegativity difference of close to 0, between 0.4-2, or >2 8. Polar covalent bonds: a) Electrons are ______________________________________________________. b) Are formed between a ___________________________ and a _______________________________. c) Are formed between two atoms that have a electronegativity difference of close to 0, between 0.4-2, or >2 9. Use the table of electronegativities provided to classify the following bonds as ionic, nonpolar covalent, or polar covalent. a) H-F ______________________ b) C-O ______________________ c) C-H ______________________ d) K-Cl ______________________ e) MgBr2 ______________________ Lewis Structures 10. Draw lewis structures for the following neutral atoms: K Ar N 11. Draw lewis structures for the following ions: K+ Ca2+ Cl- 12. Draw lewis structures for the following ionic compounds: Kl MgCl2 LiBr 13. Draw lewis structures for the following covalent compounds: CH4 H-F H2CO 14. Draw lewis structures for the following polyatomic ions: SO32- HCO3- NH4+ 15. A line in a Lewis Structure represents ____ electrons. 16. Define “lone pair”: 17. What is the total valence electron count for the following: CO2 : ______________ H3O+ : ______________ 18. Draw the resonance structure for the ion: NO3- VSEPR 19. Define and describe how VSEPR theory defines the shape of a molecule: 20. Draw the lewis structures for the molecules below, then classify their shape by using the table of shapes. CF4 CO2 NH3 Shape: Shape: Shape: 21. Use VSEPR to explain why water has a bent shape as opposed to linear. Draw a picture to support your answer. Polar molecules: 22. What is the difference between a polar molecule and a nonpolar molecule? 23. Draw a lewis structure of a water molecule. Make sure you draw the molecule with the correct shape (from #21 above). Label where the molecule is partially negative (�-) and partially positive (�+). 24. Add three (3) more water molecules to your diagram above, and draw in hydrogen bonds by using dotted lines. 25. Draw in the dipole moment as a net vector of charge (⤉) on your water molecule above. 26. Describe how a molecule’s polarity affects its: a) Solubility in water b) Intermolecular forces/surface tension c) Melting and boiling points