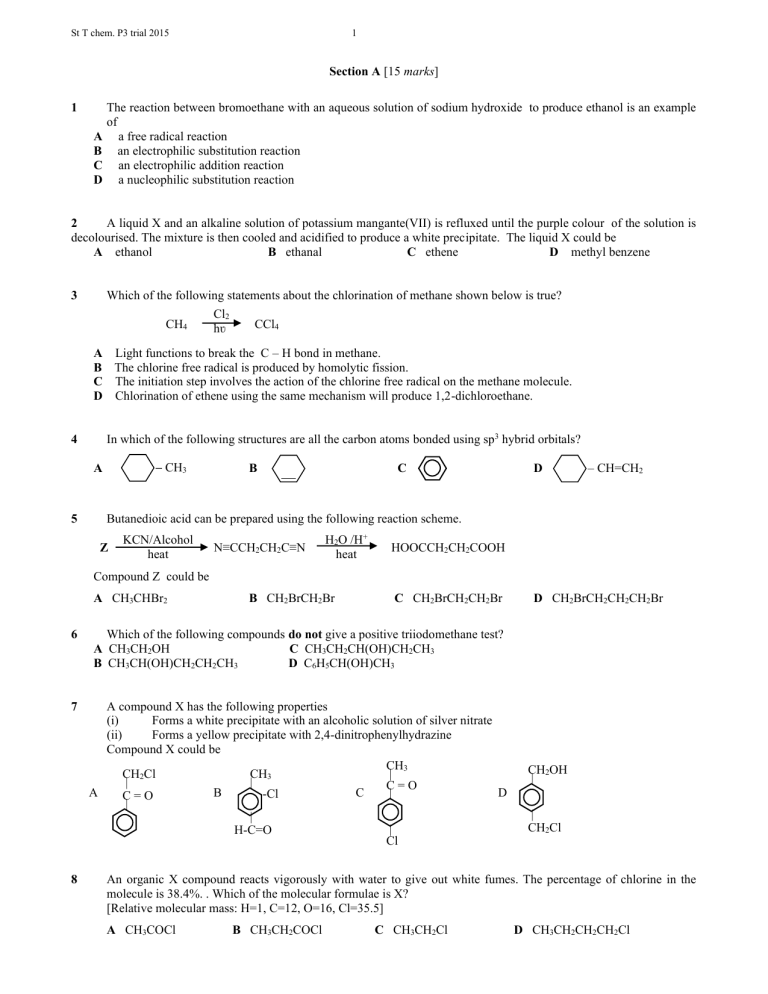
St T chem. P3 trial 2015 1 Section A [15 marks] 1 A B C D The reaction between bromoethane with an aqueous solution of sodium hydroxide to produce ethanol is an example of a free radical reaction an electrophilic substitution reaction an electrophilic addition reaction a nucleophilic substitution reaction 2 A liquid X and an alkaline solution of potassium mangante(VII) is refluxed until the purple colour of the solution is decolourised. The mixture is then cooled and acidified to produce a white precipitate. The liquid X could be A ethanol B ethanal C ethene D methyl benzene 3 Which of the following statements about the chlorination of methane shown below is true? CH4 Cl2 hʋ CCl4 A Light functions to break the C – H bond in methane. B The chlorine free radical is produced by homolytic fission. C The initiation step involves the action of the chlorine free radical on the methane molecule. D Chlorination of ethene using the same mechanism will produce 1,2-dichloroethane. In which of the following structures are all the carbon atoms bonded using sp3 hybrid orbitals? 4 – CH3 A 5 B C D – CH=CH2 Butanedioic acid can be prepared using the following reaction scheme. Z KCN/Alcohol heat N≡CCH2CH2C≡N H2O /H+ heat HOOCCH2CH2COOH Compound Z could be A CH3CHBr2 B CH2BrCH2Br C CH2BrCH2CH2Br 6 Which of the following compounds do not give a positive triiodomethane test? A CH3CH2OH C CH3CH2CH(OH)CH2CH3 B CH3CH(OH)CH2CH2CH3 D C6H5CH(OH)CH3 7 A compound X has the following properties (i) Forms a white precipitate with an alcoholic solution of silver nitrate (ii) Forms a yellow precipitate with 2,4-dinitrophenylhydrazine Compound X could be CH3 | CH2Cl CH3 | | C=O A B C D | -Cl C=O | | | H-C=O Cl 8 D CH2BrCH2CH2CH2Br CH2OH | | CH2Cl An organic X compound reacts vigorously with water to give out white fumes. The percentage of chlorine in the molecule is 38.4%. . Which of the molecular formulae is X? [Relative molecular mass: H=1, C=12, O=16, Cl=35.5] A CH3COCl B CH3CH2COCl C CH3CH2Cl D CH3CH2CH2CH2Cl St T chem. P3 trial 2015 2 9 A compound U undergoes nucleophilic substitution reaction with aqueous ammonia to produce a compound W. Ethylamine is produced when compound W is reduced by lithium tetrahydridoaluminate(III). Compound U could be A CH3CHO B CH3COCl C CH3CH2OH D CH3CH2Br 10 A diazonium salt is produced when an aqueous solution of sodium nitrate(III) in dilute hydrochloric acid is added to an acidic solution of X at low temperature. The possible structural formula of X could be A CH3 B CH2NH2 NH2 11 A B C D C CH3CH2-C – NH2 || O D OH Which of the following statements about aminoethanoic acid is not true? Its aqueous solution can conduct electrticity. It has a high melting point. It is optically active It forms zwitterion. 12 Which of the following pairs of compounds cannot be used to prepare (CH3)2C(OH)CH2CH3 ? A (CH3)2CO and CH3CH2MgBr C CH3CHO and CH3CH2MgBr B CH3COCH2CH3CH3MgBr D NaOH and CH3CH2CBr(CH3)2 13 Which of the following compounds can react with HOOC – – COOH to produce a polymer of high relative molecular mass? A CH3CH(OH)CHClCH2CH3 B HOCH2CH2CH2CH2CH3 C CH3CH(OH)CH2COOH D HOCH2CH2CH2OH 14 The repeating unit for polymer P and Q are shown: P Q H CH3 H H O | | | | || ⎯⎯ C ⎯ C ⎯⎯⎯⎯ ⎯ O − C − C − O − C − | | | | H C-O-CH3 n H H || O Which of the following statements concerning P and Q is true? Polymer P Polymer Q a polyester a polyester a condensation polymer a condensation polymer an addition polymer an addition polymer an addition polymer a condensation polymer A B C D O || −C⎯ n 15 The table below shows the dissociation constants Ka for ethanoic acid and substituted ethanoic acids at 25 oC Acid Ka/ moldm-3 CH3COOH 1.8 x 10-5 XCH2COOH 1.4 x 10-3 X2CHCOOH 3.3 x 10-2 X3CCOOH 2.0 x 10-1 Which of the following statements can be the conclusion/conclusions based on the data above? 1 Atom X is a stronger electron withdrawing atom compared to hydrogen O 2 The structure –C becomes more stable in the presence of more atoms of X in the ethanoic acid molecule O3 The more hydrogen atoms substituted by atom X in ethanoic acid the higher the acidity of the acid A 1,2 and 3 correct B 1 and 2 correct C 2 and 3 correct D Only 1 correct St T chem. P3 trial 2015 3 Section B [15 marks] 16 (a) (i) In the reaction scheme below, draw the structural formula of the compound 3-chloro-3-methylpentane, as well as the alcohol and alkene produced when 3-chloro-3-methylpentane reacts with aqueous sodium hydroxide. [3 marks] Alcohol NaOH(aq) 3-chloro-3-methylpentane Alkene (ii) State conditions for the reaction between 3-chloro-3-methylpentane with aqueous sodium hydroxide so that the major product is alcohol and alkene only. [2 marks] To produce alcohol only : ......................................................................................................................................................... ................................................................................................................................................................................................... To produce an alkene only: ...................................................................................................................................................... ................................................................................................................................................................................................... 6 (b) The table below shows the pKb values for a few nitrogen compounds. Nitrogen Compound C2H5NH2 NH3 C6 H5NH2 NH2CH2CO2H (i) pKb 3.28 4.74 9.38 11.70 Arrange the compounds above in ascending order of basic strength. [1 mark] ........................................................................................................................................................... ................... (ii) Explain the relative strength of ethanamine and phenylamine. [2 marks] ........................................................................................................................................................... ................... .............................................................................................................................................................................. ........................................................................................................................................................... ................... ........................................................................................................................................................... ................... St T chem. P3 trial 2015 17 4 Compound Y is an amide which has a relative molecular mass of 149 and percentage Composition by mass as follows: carbon, 72.5% ; hydrogen, 7.4% ; nitrogen, 9.4% , and oxygen, 10.7%. (i) Determine the molecular formula of Y [3 marks] (ii) When compound Y is refluxed with dilute sulphuric acid for a few hours and the acidic solution formed is cooled down, no white precipitate is produced. After adding aqueous ammonia to the acidic solution, a primary amine organic oil is separated out. Write the structural formula for Y and explain you answer. [3 marks] . Structural formula: Explanation: ............................................................................................................................................ ........................................................................................................................................................... ................... .............................................................................................................................................................................. ........................................................................................................................................................... ................... ........................................................................................................................................................... ................... (iii) Write an equation to show the production of the primary amine when aqueous ammonia is added to the acidic solution of Y. [1 mark] St T chem. P3 trial 2015 5 Section C [30 marks] 18 By explaining the chemical processes involved, determine the structural formula of the compounds A, B, C and D. Write equations for the reactions involved. State the types of isomerism shown by the compounds E, F G and H in (b) (a) Compounds A and B are isomers with the molecular formula C7H7Cl. The chlorine atom in compound A is easily substituted when reacted with an aqueous solution of sodium hydroxide, while the chlorine atom in compound B, does not react with aqueous solution of sodium hydroxide. [ 5 marks] (b) Compounds C and D with the molecular formula C4H10O undergoes the same dehydration reaction. Compound C produces a mixture containing three compounds E, F and G, while compound D, produces only one compound H. Compounds E, F, G and H are isomers which have the molecular formula C4H8. [10 marks] 19 (a) Give a chemical test to differentiate each of the following pairs of compounds. State the observations expected and write balanced equations for the reactions involved. (i) Pentan-2-one and Pentan-3-one (ii) Benzene andmethylbenzene [4 marks] [4 marks] (b) Cyclopentane can react with Bromine in the presence of ultra-violet light to produce 1,2 –dibromocyclopentane. (i) Name the type of reaction taking place between cyclopentane and bromine in the presence of ultra violet light. (ii) Write the reaction mechanism for the reaction. [7 marks] 20 (a) The plastic ABS, is strong and hard. ABS is made from the polymerization of three monomers, acrylonitrile (or propenenitrile) , 1,3-butadiene and phenylethene. CH2=CH-CN , CH2=CH-CH=CH2 , C6H5CH=CH2 (i) (ii) (iii) The three monomers react with one another in the ratio of 1:1:1. Draw the repeating unit for ABS. What characteristic property in ABS that enables it to be made strong and hard? Draw the structure of the polymer when it is heated in the presence of sulphur. [4 mark] (b) Determine the structural formulae of the following compounds: (i) two compounds P and Q with the following composition by mass : C,66.7% ; H,11.1% ; O, 22.2% Both these compounds have a relative molecular mass 72 and produces a yellow precipitate when reacted with an alkaline solution of iodine. (ii) three compounds R, S and T with the similar composition by mass as in (i) which do not produce a yellow precipitate with an alkaline solution of iodine. (iii) State a reagent which can be used to differentiate the two compounds in (b)(i) and the observations expected. [11 marks] END OF QUESTION PAPER





