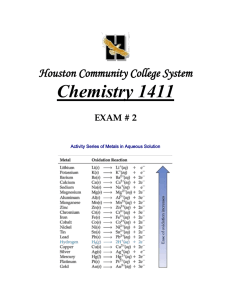
𝐷𝐷 = 𝑚𝑚 𝑉𝑉 ℎ𝑐𝑐 𝐸𝐸𝑃𝑃 = ℎ𝜈𝜈 = 𝜆𝜆 𝐴𝐴 = 𝜀𝜀𝜀𝜀𝜀𝜀 𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚 (𝑀𝑀) = 9 𝑇𝑇℉ = 𝑇𝑇℃ + 32 5 𝑐𝑐 = 𝜆𝜆𝜆𝜆 𝑀𝑀1 𝑉𝑉1 = 𝑀𝑀2 𝑉𝑉2 𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚 𝑜𝑜𝑜𝑜 𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠 𝑙𝑙𝑙𝑙𝑙𝑙𝑙𝑙𝑙𝑙𝑙𝑙 𝑜𝑜𝑜𝑜 𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠𝑠 % 𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦 = 𝑇𝑇𝐾𝐾 = 𝑇𝑇℃ + 273.15 1 1 2 − 2� 𝑛𝑛𝑓𝑓 𝑛𝑛𝑖𝑖 𝑍𝑍 = 𝑛𝑛𝑛𝑛𝑛𝑛𝑛𝑛𝑛𝑛𝑛𝑛 𝑜𝑜𝑜𝑜 𝑝𝑝𝑝𝑝𝑝𝑝𝑝𝑝𝑝𝑝𝑝𝑝𝑝𝑝 𝑃𝑃𝑃𝑃 = 𝑛𝑛𝑛𝑛𝑛𝑛 Δ𝐸𝐸 = −𝑍𝑍 2 𝑅𝑅𝐻𝐻 � 𝑎𝑎𝑎𝑎𝑎𝑎𝑎𝑎𝑎𝑎𝑎𝑎 𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦 × 100 𝑡𝑡ℎ𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒𝑒 𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦𝑦 ∘ ∆𝐻𝐻𝑟𝑟𝑟𝑟𝑟𝑟 ≈ � 𝐵𝐵𝐵𝐵(𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏 𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏) − � 𝐵𝐵𝐵𝐵(𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏𝑏 𝑓𝑓𝑓𝑓𝑓𝑓𝑓𝑓𝑓𝑓𝑓𝑓) Soluble Ionic Compounds 1. Group 1A(1) ions (Li+, Na+, K+, etc.) and ammonium (NH4+) 2. NO3–,CH3CO2–, and ClO4– 3. Cl–, Br–, I– except with Ag+, Pb2+, Cu+, and Hg22+ 4. SO42– except with Ca2+, Sr2+, Ag+, and Pb2+. Bond Enthalpies: Bond C–C C=C C≡C C–H O–H H–H C–O C–N C – Cl Bond Energy (kJ/mol) 347 614 839 413 467 432 358 305 339 Avogadro’s Number: NA = 6.022 x 1023 mol-1 Speed of light: c = 2.99792458 x 108 m/sec Planck’s constant: h = 6.626 x 10-34 J sec Rydberg constant: RH = 2.18 x 10-18 J 1 amu = 1.66 x 10-27 kg R = 0.0821 L⋅atm/K⋅mol R = 8.314 J/ K⋅mol STP: 1.00 atm & 0˚C 1 cal = 4.184 J 1 ft = 12 inches = 0.3048 m 1 km = 0.62137 miles 1 atm = 760 torr = 1.01325 bar 1 eV = 1.602 x 10-19 J 1 cm3 = 1 mL Insoluble Ionic Compounds 1. OH– except with Group 1A(1), NH4+, and larger Group 2A(2)(beginning with Ca2+) 2. CO32– except with Group 1A(1) and NH4+ 3. S2– except with Group 1A(1) and NH4+ 4. C2O4–2 except with Group 1A(1), and NH4+. 5. PO43– except with Group 1A(1) beginning with Na+, and NH4+. Bond C = O (in CO2) C=O C≡O O=O N–H N≡N N – Cl N–O C≡N Bond Energy (kJ/mol) 799 745 1070 498 391 945 200 201 891