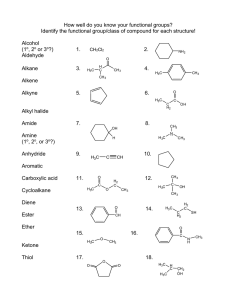
Unit-2 Nomenclature 2/4/2023 1 organic compounds are grouped into classes based on their functional groups. A functional group is an atom or a group of atoms within a molecule that serves as a site of chemical reactivity. The most important functional groups are shown in the following table, 2/4/2023 2 2/4/2023 3 2/4/2023 4 2/4/2023 5 Hydrocarbons are compounds that only contain carbon and hydrogen atoms, and they can be classified as follows depending on the bond types that are present within the molecules. 2/4/2023 6 Nomenclature of alkanes Alkanes are hydrocarbons having no double or triple bond . have the general molecular formula CnH2n+2 and are called saturated hydrocarbons. Saturated hydrocarbons contain only single bonds, and are also commonly referred to as aliphatic or acyclic alkanes (alkanes without rings). Thus, the alkane family is characterized by the presence of tetrahedral carbon (sp3) atoms. Methane (CH4) and ethane (C2H6) are the first two members of the alkane family. A group derived from an alkane by removal of one of its hydrogen atoms is known as an alkyl group, for example the methyl group (CH3--) from methane (CH4) and the ethyl group (CH3CH2--) from ethane (CH3CH3). 2/4/2023 7 In general, organic compounds are given systematic names by using the order: prefix–parent–suffix Where: prefix indicates how many branching groups are present, parent indicates how many carbons are in the longest chain and suffix indicates the name of the family. 2/4/2023 8 The systematic name of an alkane is obtained using the following rules: 1. Determine the number of carbons in the longest continuous carbon chain. This chain is called the parent hydrocarbon. 2/4/2023 9 2. Number the chain so that the substituent gets the lowest possible number 3. If more than one substituent is attached to the parent hydrocarbon, the chain is numbered in the direction that will result in the lowest possible number in the name of the compound. The substituents are listed in alphabetical (not numerical) order, with each substituent getting the appropriate number. A number and a word are separated by a hyphen; numbers are separated by a comma. 2/4/2023 10 If two or more substituents are the same, the prefixes “di,” “tri,” and “tetra” are used to indicate how many identical substituents the compound has. The prefixes di, tri, tetra, sec, and tert are ignored in alphabetizing substituent groups, but the prefixes iso, neo, and cyclo are not ignored. 2/4/2023 11 4. When both directions lead to the same lowest number for one of the substituents, the direction is chosen that gives the lowest possible number to one of the remaining substituents. 5. If the same substituent numbers are obtained in both directions, the first group cited receives the lower number. 2/4/2023 12 6. If a compound has two or more chains of the same length, the parent hydrocarbon is the chain with the greatest number of substituents. Some substituents have only a systematic name. 2/4/2023 13 Nomenclature of Cycloalkanes Cycloalkanes are alkanes with their carbon atoms arranged in a ring. have two fewer hydrogens than an acyclic (noncyclic) alkane with the same number of carbons. are named by adding the prefix “cyclo” to the alkane name that signifies the number of carbon atoms in the ring. 2/4/2023 14 The rules for naming cycloalkanes resemble the rules for naming acyclic alkanes: 1. In the case of a cycloalkane with an attached alkyl substituent, the ring is the parent hydrocarbon unless the substituent has more carbon atoms than the ring. In that case, the substituent is the parent hydrocarbon and the ring is named as a substituent. There is no need to number the position of a single substituent on a ring. 2. If the ring has two different substituents, they are cited in alphabetical order and the number 1 position is given to the substituent cited first. 2/4/2023 15 3. If there are more than two substituents on the ring, they are cited in alphabetical order. The substituent given the number 1 position is the one that results in a second substituent getting as low a number as possible. If two substituents have the same low number, the ring is numbered—either clockwise or counterclockwise—in the direction that gives the third substituent the lowest possible number. 2/4/2023 16 1 CH3 2 CH3 H3C 1,2-Dimethylcyclopentane not 1,5-dimethylcyclopentane 1 3 CH3 1,3-Diethylcyclohexane not 1,5-diethylcyclohexane CH3 CH3 2 1 CH3 7 3 1 2 4 5 6 9 8 5-Cyclopentyl-4-methylnonane not 5-Cyclopentyl-6-methylnonane 1,1,2-Trimethylcyclohexane not 1,2,2-Trimethylcyclohexane Give the IUPAC name for each of the following compounds: a) 2/4/2023 b) H3C H3C c) CH2CH3 17 Nomenclature of Alkyl Halides Formed when a hydrogen of an alkane is replaced by a halogen. classified as primary, secondary, or tertiary, depending on the carbon to which the halogen is attached. Primary alkyl halides have a halogen bonded to a primary carbon, secondary alkyl halides have a halogen bonded to a secondary carbon, and tertiary alkyl halides have a halogen bonded to a tertiary carbon. The common names of alkyl halides consist of the name of the alkyl group followed by halide (i.e., fluoride, chloride, bromide, iodide). 2/4/2023 18 In the IUPAC system, alkyl halides are named as substituted alkanes: Haloalkanes. 2/4/2023 19 Nomenclature of Alkenes The systematic (IUPAC) name of an alkene is obtained by replacing the “ane” ending of the corresponding alkane with “ene.” Most alkene names need a number to indicate the position of the double bond. 1. Number the longest continuous chain containing the functional group in the direction that gives the functional group suffix the lowest possible number 2/4/2023 20 2. The name of a substituent is cited before the name of the longest continuous chain containing the functional group, together with a number to designate the carbon to which the substituent is attached. Notice that the chain is still numbered in the direction that gives the functional group suffix the lowest possible number. 3. If the same number for the alkene functional group suffix is obtained in both directions, the correct name is the name that contains the lowest substituent number. 2/4/2023 21 4. In cyclic alkenes, a number is not needed to denote the position of the functional group, because the ring is always numbered so that the double bond is between carbons 1 and 2. 5. If both directions lead to the same number for the alkene functional group suffix and the same low number(s) for one or more of the substituents, then those substituents are ignored and the direction is chosen that gives the lowest number to one of the remaining substituents. 2/4/2023 22 Draw the structure for each of the following compounds: a. 3,3-dimethylcyclopentene b. 6-bromo-2,3-dimethyl-2-hexene Give the systematic name for each of the following compounds: Give the IUPAC name for each of the following compounds: Br CH3CH2 a) CH3CHCH2CH=CCH2CHCH3 CH3 b) CH2CH3 CH3CH2CH2CH2CH c) 2/4/2023 CH3 CH CH CH2 23 Nomenclature of Alkynes Alkynes are hydrocarbons that contain a carbon–carbon triple bond. A triple bond consists of a bond and two bonds. The general formula for the alkynes is CnH2n-2. Terminal CH groups are called acetylenic hydrogens. If the triple bond has two alkyl groups on both sides, it is called an internal alkyne CH3CH2CCH CHCH 4 Ethyne (acetylene) CH3CH2CCCH3 2 1 3 5 4 3 21 2-Pentyne (ethylmethylacetylene) Internal alkyne 1-Butyne (ethylacetylene) Terminal alkyne The IUPAC nomenclature of alkynes is similar to that for alkanes, except the –ane ending is replaced with –yne. CH3 CH3 CH3CCCHCH2CH3 1 2 3 4 5 6 4-Methyl-2-hexyne Br CH3CHCCCHCH2CH3 1 2 3 4 5 6 (CH3)3CCCCH3 7 5-Bromo-2-methyl-3-heptyne 4,4-Dimethyl-2-pentyne Question Write structural formulas and give the IUPAC names for all the alkynes of molecular formula C5H2/4/2023 8. 24 Classification of carbon substitution: A carbon atom is classified as primary (1°), secondary (2°), tertiary (3°) and quaternary (4°) depending on the number of carbon atoms bonded to it. Primary carbon Primary carbon H3C CH2 CH2 CH3 CH3 CH C Tertiary carbon Secondary carbon 2/4/2023 CH3 CH3 Quaternary carbon Primary carbon 25 Alcohols The functional group of an alcohol is the hydroxyl (OH) group. Therefore, an alcohol has the general formula ROH. The simplest and most common alcohols are methyl alcohol (CH3OH) and ethyl alcohol (CH3CH2OH). An alcohol may be acyclic or cyclic. It may contain a double bond, a halogen atom or additional hydroxyl groups. Alcohols are usually classified as primary (1°), secondary (2°) or tertiary (3°). H H CH3CH2 C OH H Propanol Propyl alcohol (1) 2/4/2023 H3C C H OH CH3 Isopropanol 2-Propanol (2) H3C C OH OH CH3 tert-Butanol 2-Methyl-2-propanol (3) Phenol 26 Nomenclature of Alcohols Generally, the name of an alcohol ends with -ol. An alcohol can be named as an alkyl alcohol, usually for small alkyl groups e.g. methyl alcohol and ethyl alcohol. The longest carbon chain bearing the OH group is used as the parent; the last -e from this alkane is replaced by an -ol to obtain the root name. CH3 OH OH H3CCHCHCH2Br 4 2 3 1 1-Bromo-3-methyl-2-butanol (The OH is at C-2 of butane) HO CH2CH2CH3 CH3CH2CHCH2CH2Cl 5 2 3 4 1 1-Propylcyclopentanol 1-Chloro-3-pentanol (The OH is at C-3 of pentane) OH CH3CH2CHCH2CO2H 5 4 2 3 1 3-Hydroxypentanoic acid 1 1 OH OH 2 5 4 3 2 Pent-4-en-2-ol 1 2 3 CH2CHCH3 OH OH 1,2-Ethane diol (Ethylene glycol) 2/4/2023 OH 1,2-propane diol (Propylene glycol) 3 Cl 1 CH2CH2 OH 5 H2C=CHCH2CHCH3 Br 3-Bromo-5-chlorocyclohexanol OH 6 5 4 3 2 1 OH 3,4-Hexanediol 27 Name the following compounds according to the IUPAC system: OH a) OH 2/4/2023 c) b) OH 28 Ethers Ethers are also organic relatives of water, where alkyl groups replace both hydrogen atoms. Thus, ethers have two hydrocarbons bonded to an oxygen atom. The simplest and most common ethers are diethyl ether and tetrahydrofuran (THF), which is a cyclic ether. O C2H5OC2H5 Diethyl ether (Ether) Tetrahydrofuran (THF) Ethers are relatively unreactive towards most reagents, so they are frequently used as solvents in organic reactions. A few other common ether solvents are shown below. CH3 CH3OCH2CH2OCH3 1,2-Dimethoxyethane (DME) 2/4/2023 CH3OCCH3 O CH3 O Methyl tert-butyl ether (MTBE) 1,4-Dioxane 29 Nomenclature of Ethers Ethers can be symmetrical, where the two alkyl groups are the same, or unsymmetrical, where the two alkyl groups are different H3COCH3 H3COC2H5 Dimethylether (symmetrical) Ethyl methyl ether (unsymmetrical) In the nomenclature of ethers, either the suffix -ether or the prefix alkoxy- is used. CH3 C2H5OC2H5 Diethyl ether (Ethoxyethane) 2/4/2023 CH3OCCH3 CH3 Methyl tert-butyl ether (2-Methyl-2-methoxypropane) 30 Three membered cyclic ethers are known as epoxides. They are just a subclass of ethers containing a three-membered oxirane ring (COC unit). Cyclic ethers have the prefix epoxy- and suffix -alkene oxide. Five membered and six membered cyclic ethers are known as oxolane and oxane, respectively. H2C CH2 CH3CH CH2 CH2 CH3CH2CH O O O Ethylene oxide (Epoxy ethane) Propylene oxide (1,2-Epoxy propane) Butylene oxide (1,2-Epoxy butane) O O O O Oxirane ring (epoxide) Oxolane (THF) Oxane Question Write the structure of each of the following ethers. (a) Chloromethyl methyl ether (b) 2-(Chloromethyl)oxirane (c) 3,4-Epoxy-1-butene (2-vinyloxirane) 2/4/2023 31 Amines Amines are nitrogen containing compounds, the functional group is an amino group (NH2). They are organic relatives of ammonia, where one or more of the hydrogen atoms of ammonia are replaced by alkyl group(s) Amines are classified as primary (1°), secondary (2°), tertiary (3°) or quaternary (4°) depending on how many alkyl groups are attached to the N atom. CH3 CH3CH2CH2NH2 Propylamine (1 amine) CH3NHCH3 CH3NCH3 Dimethylamine (2 amine) Trimethylamine (3 amine) CH3 CH3NCH3 CH3 Tetramethylamine (4 amine) Aliphatic amines are named according to the alkyl group or groups attached to nitrogen with the suffix -amine CH3 H2NCH2CH2NH2 Ethylenediamine (1° amine) 2/4/2023 C2H5NHCH3 Methylethylamine (2° amine) CH3NHCH(CH2)3CH3 2-(N-Methylamino)hexane (2 amine) 32 If other substituents are attached to the nitrogen atom, they are indicated by the prefix Nbefore the name of the substituents. The simplest aromatic amine is aniline (C6H5NH2), where nitrogen is attached directly to a benzene ring. Provide a structural formula for each of the following compounds: (a) 2-Ethyl-1-butanamine (c) Tetraethylammonium hydroxide (e) 2,2-Dimethyl-1,3-propanediamine 2/4/2023 (b) N-Ethyl-1-butanamine (d) N-Allylcyclohexylamine 33 Nitriles a triple bond between a carbon and a nitrogen atom. The functional group in nitriles is the cyano (C≡N) group, and they are often named as cyano compounds. H3CCN CH3CH2CN Ethanenitrile Acetonitrile Propanenitrile The IUPAC requires nitriles to be named on the basis of the name of the alkanes, with the suffix -nitrile. OCH3 CN Benzonitrile 2/4/2023 H3CCHCH2CH2CH2CN 5-Methoxyhexanenitrile 34 Aldehydes and Ketones A carbonyl functional group (C=O) is a carbon double bonded to an oxygen atom. An acyl functional group (RC=O) consists of a carbonyl group attached to an alkyl or an aryl group. O O C Carbonyl group R Acyl group (R = alkyl or aryl) O O H C C H Formaldehyde H3C C O H Ethanal Acetaldehyde H3C C CH3 Propanone Acetone In the IUPAC nomenclature of Aldehydes, the -e of the alkane is replaced with -al, e.g. ethanal (the parent alkane is ethane). Ketones are named by replacing the -e ending of the alkyl name with -one, e.g. propanone (the parent alkane is propane). 2/4/2023 35 If the aldehyde group is a substituent on a ring, the suffix -carbaldehyde is used in the name. O CH 3 O H3C C CCH3 Cl O CH3 H 4-Chlorocyclohexanone 3-Methyl-2-butanone Cyclohexanecarbaldehyde An aldehyde or ketone group can also be named as a substituent on a molecule with another functional group as its root. The aldehyde carbonyl is given the prefix formyl-, and the ketone group is named oxowith a number to show its position in the molecule. O C2H5 C O CH2CH 3-Oxopentanal O CH H3C O O C CH2CH 3-Oxobutanoic acid H3C C CH2CH2H 4-Hydroxy-2-butanone O O C O CH3 C COH O Acetophenone Benzophenone 2-Formylbenzoic acid 2/4/2023 36 Write a structural formula for each of the following. (a) 2,2,2-trichloroethanal (c) (E)-2-butenal 2/4/2023 (b) 2,2-dimethylpropanal (d) 4-hydroxy-4-methyl-2-pentanone 37 Carboxylic Acids Carboxylic acid is an organic acid that has an acyl group (RC=O) linked to a hydroxyl group (OH). In a condensed structural formula, a carboxyl group may be written as CO2H, and a carboxylic acid as RCO2H. O O COH R COH CO2H or COOH RCO2H or RCOOH Carboxylic acid Carboxyl group O O O HCOH H3C COH Methanoic acid Formic acid Ethanoic acid Acetic acid COH Benzenecarboxylic acid Benzoic acid The root name is based on the longest continuous chain of carbon atoms bearing the carboxyl group. The -e is replaced by -oic acid. The chain is numbered starting with the carboxyl carbon atom. The carboxyl group takes priority over any other functional groups as follows: carboxylic acid > ester > amide > nitrile > aldehyde > ketone > alcohol > amine > alkene > alkyne. 2/4/2023 38 O O O CH3CH2COH CH3CH2CH2CH2COH CH3CH2CH2COH Propanoic acid Propionic acid Pentanoic acid Valeric acid Butanoic acid Butyric acid O O O H2C=CHCOH CH3CH2CHCOH Propenoic acid Acrylic acid OMe H2N 2-Methoxybutanoic acid OH 4-Aminobutanoic acid Cycloalkanes with carboxyl substituents are named as cycloalkanecarboxylic acids. Unsaturated acids are named using the name of the alkene with -e replaced with -oic acid. O CO2H HOCCHCH3 CH3 2-Cyclohexylpropanoic acid 3-Methylcyclohexanecarboxylic acid C2H5 H H3C CH2CO2H (E)-4-Methyl-3-hexenoic acid Aliphatic dicarboxylic acids are named by simply adding the suffix –dioic acid to the root name. Br O HO OH O 2/4/2023 Br 3,4-Dibromohexanedioic acid 39 Provide a structural formula for each one on the basis of its systematic name. (a) 2-Hydroxy-2-phenylethanoic acid (b) (b) 10-Undecenoic acid (c) 3,5-Dihydroxy-3-methylpentanoic acid (d) (E)-2-Methyl-2-butenoic acid (e) 2-Hydroxy-1,2,3-propanetricarboxylic acid (f) 2-(p-Isobutylphenyl)propanoic acid 2/4/2023 40 Carboxylic Acid Derivatives Carboxylic acid derivatives are compounds that possess an acyl group (RC=O) linked to an electronegative atom, e.g. Cl, CO2R, OR or NH2. They can be converted to carboxylic acids via simple acidic or basic hydrolysis. The important acid derivatives are acid chlorides, acid anhydrides, esters and amides. O R C Cl Acid chloride RCOCl 2/4/2023 R O O C OCR Acid anhydride (RCO)2O O R C O OR Ester RCO2R RCNHR Amide RCONH2 41 Nomenclature of Acid Chlorides Acid chlorides are named by replacing the -ic acid ending with -yl chloride or replacing the carboxylic acid ending with -carbonyl chloride. O O O CH3CCl CH3CH2CCl CH3CH2CH2CCl Ethanoyl chloride Acetyl chloride Propanoyl chloride Butanoyl chloride O CH3CH2CH2CH2CCl Pentanoyl chloride 2/4/2023 Br O CH3CH2CHCCl O O Cl Cl 3-Bromobutanoyl chloride Cyclohexanecarbonyl Benzoyl chloride chloride 42 Acid Anhydrides The functional group of an acid anhydride is two acyl groups bonded to an oxygen atom. These compounds are called acid anhydrides or acyl anhydrides, because they are condensed from two molecules of carboxylic acid by the loss of a water molecule. H3C C O O O OCCH3 Ethanoic anhydride Acetic anhydride H3C C O OCCH2CH3 Acetic propanoic anhydride Symmetrical acid anhydrides are named by replacing the -acid suffix of the parent carboxylic acids with the word anhydride. Mixed anhydrides that consist of two different acid-derived parts are named using the names of the two individual acids with an alphabetical order. O O CH3CH2 C OCCH2CH3 Propanoic anhydride 2/4/2023 O CH3CH2 C O OCCH2CH2CH3 Butanoic propanoic anhydride 43 Esters The functional group of an ester is an acyl group bonded to an alkoxy group (OR). O O H3CCOCH3 O H3CCOCH2CH3 H3CCOCH2CH2CH3 Ethyl acetate Ethylethanoate Methyl acetate Methylethanoate Propyl acetate Propylethanoate The names of esters originate from the names of the compounds that are used to prepare them. The first word of the name comes from the alkyl group of the alcohol, and the second part comes from the carboxylate group of the carboxylic acid used. A cyclic ester is called a lactone, and the IUPAC names of lactones are derived by adding the term lactone at the end of the name of the parent carboxylic acid. O O CH3CH2COCH2(CH3)2CH3 Butyl propanoate Isopentyl acetate O O OC(CH3)3 t-Butylcyclohexanecarboxylate 2/4/2023 H3CCOCH2CH2CH(CH3)2 O OC2H5 Ethyl benzoate O 4-Hydroxybutanoic acid lactone 44 Amides The functional group of an amide is an acyl group bonded to a nitrogen atom. The simplest members of this family are formamide (HCONH2) and acetamide (CH3CONH2). O O O HCNH2 H3CCNH2 C2H5CNH2 Methanamide Formamide Ethanamide Acetamide Propanamide Amides are usually classified as primary (1°) amide, secondary (2°) or N-substituted amide, and tertiary (3°) or N,N-disubstituted amide. O O RCNH2 Primary amide (1 O RCNHR RCNR2 Secondary (2) or N-substituted amide Tertiary (3) or N,N-disubstituted amide Amides are named by replacing the -oic acid or -ic acid suffix of the parent carboxylic acids with the suffix -amide, or by replacing the -carboxylic acid ending with -carboxamide. Alkyl groups on nitrogen atoms are named as substituents, and are prefaced by N-or N,N-, followed by the name(s) of the alkyl group(s). O H3CCNHCH2CH3 N-Ethylethanamide 2/4/2023 O O CNH2 CNH2 O HCN(CH3)2 N,N-Dimethylformamide Cyclohexanecarboxamide DMF Benzamide 45 Provide a structural formula for each of the following compounds: a) 3,3-dimethylheptanoic acid c) 4-methyl-3-pentenoyl chloride e) N,N-dimethyl-2-ethylbutanamide 2/4/2023 b) 3-chloro-1,1-cyclopentanedicarboxylic acid d) Ethyl para-methoxybenzoate 46