Chemistry Reactions Worksheet
advertisement

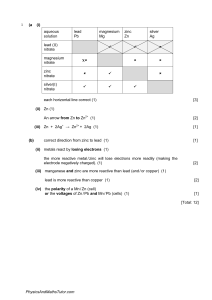
4. Answer all three portions in this part. Give the formulas to show the reactants and the products for the three following chemical reactions. Each reaction occurs in aqueous solution unless otherwise indicated. Represent the substances in solution as ions if the substance is extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction. In all cases a reaction occurs. You need to balance the chemical equation. Then answer a question about the reaction. Example: A strip of magnesium is added to a solution of silver nitrate: Mg + 2Ag+ Mg2+ + 2Ag w a. A solution of sodium hydroxide is added to a solution of lead (II) nitrate If 1.0 L of 1.0 M solutions of both reactants are mixed together, how many moles of product(s) will be produced? Assume reaction goes to completion. b. Excess nitric acid is added to solid calcium carbonate Briefly explain why statues made of marble(calcium carbonate) displayed outdoors in urban areas are deteriorating. c. A solution containing silver (I) ion(oxidizing agent) is mixed with a solution containing iron (II) ion(reducing agent) If the contents of the reaction mixture described above are filtered, what substance(s), if any, would remain on the filter paper.