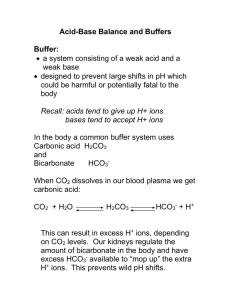
One Headache After Another: Biochemistry Edition by Ann Taylor, Chemistry Department, Wabash College William Clif, Biology Department, Niagara University Part I – Blood Work “Chris. Michelle. You go on… I’ll catch up in a minute.” Mary hid her fear as she struggled to catch her breath. She had been so excited to go hiking with her children. Chris and Michelle were both home on fall break, and Mary had been looking forward to the family time. But she felt like she was hyperventilating, and now her chest was starting to hurt. “What, we’ve fnally outlasted you on the trails?” Chris joked as he looked over his shoulder. “Mom, are you ok? You look awful.” “Don’t panic, but I think I need to go to the hospital.” “Michelle! Go get the car!” Chris yelled ahead. “Please don’t panic—I just want to get it checked out.” “Michelle!” After what seemed like an eternity, they arrived at the hospital. As Chris and Michelle helped their mother into the waiting room, the ER attendant noticed them and ushered the family into a small room. Dr. Rosen promptly took Mary’s medical history and ordered an EKG, chest x-ray, blood tests, and urinalysis. Te tests were completed in short order, and about an hour later, Dr. Rosen appeared in the exam room. “I have some good news, and a little bad news. Te good news is…” “Dr. Rosen to the ER, Code Blue. Stat!” said the voice on the PA. “Sorry, that’s my cue. But don’t worry, you’ll be fne…once we get you of of the Topamax®. Tis might take a while, so I’m leaving your chart. I hope I’ll be back to review the test results with you, but it might be someone else,” said the doctor as the door swung shut. “Mom, isn’t Topamax your migraine medicine?” asked Michelle. “Yes. It was originally designed as an epilepsy treatment, but I take it to prevent those migraines that used to put me in bed all day. How could it be causing this?” Mary wondered out loud. Chris broke the tension, saying, “Tese are your records, right? Let’s see what we can fgure out.” He fipped open her chart. On the top of the pile were Mary’s blood test results: “One Headache After Another: Biochemistry Edition” by Taylor & Clif Page 1 Hemoglobin (g/dL) Hematocrit (%) Creatine kinase cardiac isozyme Creatine kinase MM isozyme Creatinine pH pCO2 (mmHg) pCO2 (mM) pO2 (mmHg) HCO3- (mM) Glucose (mg/dL) Normal range 12.0–15.0 36–44 0–3.9% 96–100% 0.5–1.4 7.35–7.45 38–52 20–27 70–100 19–25 90–140 Mary’s results* 12.8 37.5 1 99 0.9 7.31 18.1 9.5 121.0 8.9 112 *Based upon data from J.E. Burmeister, R.R. Pereira, E.M. Hartke, and M. Kreuz. 2005. Neuro-Psiquiatr 63(2-b): 532–534. Question 1. What appears to be Mary’s problem(s), from the results of her blood work? What sort of change has occurred in her acid-base balance? If there is more than one problem in her blood work results, is there a relationship among those factors? “One Headache After Another: Biochemistry Edition” by Taylor & Clif Page 2 Part II – Bufers Dr. Rosen reappeared in the cubicle. “Sorry for the interruption, and for leaving you in suspense. Te good news is that you did not have a heart attack. But I do think you’re having an unusual reaction to Topamax. It appears to be inhibiting your renal carbonic anhydrase, leading to metabolic acidosis. We’ll be giving you an IV, and you should taper of your Topamax under your family physician’s supervision.” Dr. Rosen checked his pager and said, “Sorry, it’s one of those days. Again, you’ll be all right, and the nurse will be with you shortly to start the IV.” He turned on his heel and left just as quickly as he had appeared. “Mom, don’t worry,” said Chris. “I’m going to call one of my friends studying biochemistry. He should be able to help us fgure this out.” Chris dials his cell phone; yours rings. After you complete the following questions, write a one- or twoparagraph explanation to help Chris understand what’s going on with his mom. Questions 2a. Bicarbonate is one of the main bufers in the blood. Provide a defnition of a bufer. 2b. Give an equation that allows one to calculate the pH of a bufer solution. 2c. Te pKa’s for carbonic acid and bicarbonate at 37°C are 3.83 and 10.25, respectively. i. Write the equation for each of these equilibria. ii. Below is a titration curve for carbonic acid. Indicate key points on the curve, such as equivalence points and bufer regions. At what pH(s) would you expect carbonic acid species to have efective bufering capacity? Explain. “One Headache After Another: Biochemistry Edition” by Taylor & Clif Page 3 iii. Calculate the ratio of bicarbonate to carbonic acid at pH 7.4, using the pKa value(s) in 2c. iv. What is unusual about these ratios? Would you expect this to be an efective bufer system at pH 7.4? Explain. 2d. Bicarbonate does bufer the blood because carbonic acid is generated from dissolving CO2 (g) in liquid water: CO2 (g) + H2O (l) H2CO3 (aq) pKeq° = 2.52 at 37°C Tis reaction is catalyzed by the enzyme carbonic anhydrase. Consequently, the reaction that really represents what’s happening when bicarbonate bufers the blood is: CO2 (g) + H2O (l) HCO3- (aq) + H+ (aq). Calculate the equilibrium constant for this reaction (Hint: use Hess’s law and one of the equilibrium expressions from question 2ci above). Would you expect this to be an adequate bufer system at physiological pH? Explain. “One Headache After Another: Biochemistry Edition” by Taylor & Clif Page 4 Part III – Regulation Questions 3. Blood pH is normally regulated by the respiratory system to control the level of CO2 (g) and by the urinary system to control the levels of HCO3- and non-volatile acids in the blood. Metabolism Tissue CO2 Tubular cell CO2 CO2 + H2O Blood Plasma Alveolus (in lungs) Tubular lumen Proximal renal Capillary endothelium CO2 + H2O H+ + RBC Carbonic anhydrase HCO3- Cl HCO3-+ H+ Na + Cl- CO2 + H2O Carbonic anhydrase H++ Na Carbonic anhydrase + HCO3- HCO3- Given the overall reaction, CO2 (g) + H2O (l) H2CO3 (aq) HCO3- (aq) + H+ (aq), what efect would each of the following have on blood pH? Explain with LeChatlier’s principle. a. Hyperventilating b. Holding your breath for an extended period of time c. Excessive ingestion of baking soda, HCO3d. Inhibiting renal carbonic anhydrase 4. Mary has an acid-base disturbance that is being compensated for with another body system. Identify the type of disturbance and trace out the physiological pathway responsible for the compensating action. 5. For each of the treatments listed below, indicate whether you would recommend it (yes) or not (no) to correct Mary’s problem? Explain what efect each treatment would have upon her blood pH. a. Breathing in a paper bag b. An IV containing pure HCO3- in an isotonic solution c. Holding her breath for as long as she can d. An IV containing pure CO3-2 in an isotonic solution • Title block illustration: ©Dawn Hudson - Fotolia.com. Case copyright held by the National Center for Case Study Teaching in Science, University at Bufalo, State University of New York. Originally published December 28, 2009. Please see our usage guidelines, which outline our policy concerning permissible reproduction of this work. “One Headache After Another: Biochemistry Edition” by Taylor & Clif Page 5