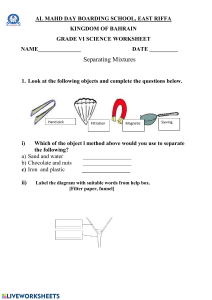
Elements, Compound and Mixtures “Indivisible” Dalton’s Atomic Model Anything that have mass and volume. Everything is made up of atoms Atoms of the same element are always the same. Atoms cannot be created or destroyed Mixture and Compound What are the different ways of separating mixtures? Magnetism Filtration Evaporation Chromatography Distillation Magnetism If one component of the mixture has magnetic properties, you could use a magnet to separate the mixture. Iron, nickel, and cobalt are all materials that are magnetic. Not all metals are magnetic: gold, silver, and aluminum are examples of metals that are not magnetic. Example of magnetism Using a magnet to separate nails from wood chips. Filtration Used when separating a solid substance from a fluid (a liquid or a gas) by passing a mixture through a porous material such as a type of filter. Works by letting the fluid pass through but not the solid. Examples of filters: coffee filter, cloth, oil filter, even sand! Example of filtration: Using a coffee filter to separate the coffee flavor from the coffee beans. Evaporation Allowing the liquid to evaporate, leaving the soluble solid behind. Example: heating sugar water. The water evaporates and the sugar crystals are left behind. Chromatography Used to separate dissolved substances in a solution from each other. Stationary Phase Separatio n Mobile Phase Mixture Componen ts Example of chromatography: Using chromatography paper to separate ink into it’s original components. Distillation Separation of liquid mixture by using the difference in boiling point. Eg Water and Ethanol The Periodic Table Electrolysis Process by which an electric current is passed through a substance to cause a chemical change. The chemical change is one in which the substance loses or gains an electron. Diagram of Electrolysis Electrolysis of Copper Chloride For more detail contact us Electrolysis of Water Water is split with electricity to produce hydrogen gas and oxygen gas. Electrolysis of Water