A- LEVEL ORGANIC
CHEMISTRY
Chemistry
benzene
The
25.1
ring
kekule
benzene
'
:
structure
benzene
of
H
I
colourless
-
state
-
highly
-
C
/
{
+
also
{
H
( does
not
]
shown
as
It
water
solvent
a
as
with
mix
H
-
1
I
well
works
-
-
liquid
→
immiscible
-
Cy
H
volatile
-
toxic
carcinogen
-
hazardous
-
formulae
-
found
is
Benzene
.
ring
-
Arenes
In
.
hexagon
,
skin
in
compounds
many
made
many
hydrocarbon
:
general
of
group
found
-
.
through
vanilla
in
functional
important
-
absorbed
HG
(•
:
benzene
-
be
can
-
of
6
carbon
compounds
containing
benzene
of
compounds
organic
that
one
are
bonded
atoms
are
known
commercially
as
aryl
particular
a
in
important
benzene
more
or
together
:
e.
g.
as
way
medicines
,
dyes
and
plastics
rings
compounds
or
aromatic
compounds
e.
g.
chlorobenzene
,
which
is
halogenoarenes
-
-
'
•
in
kekule
benzene
kekule
this
's
would
's
structure
the
found
to
was
structure
produce
would
a
hexagonal
be
suggest
distorted
a
ring
planar
three
,
contained
perfectly
shorter
hexagonal
double
three
double
symmetrical
C=C
bonds
C.
=
C
bonds
molecule
and
three
longer
C- C
single
bonds
in
the
ring
one
of
the
An
introduction
Functional
Groups
Groups
Functional
is
atom
an
AL)
:
group
or
Compounds (
Organic
Of
Chemistry
Organic
to
of
atoms
molecule
organic
an
in
determine
that
,
characteristic
its
and
chemical
physical
properties
A-BENES
-
•
hydrocarbon
containing
aromatic
are
arenes
one
that
compounds
benzene
more
or
contain
rings
BENZENE RING
a
functional
arene
chemical
properties
due
-
under
-
the
however
delocalised
the
to
because
this
delocahsed
the
different
very
is
Physical Properties
-
-
-
benzene
the
it
Van
presence
is
not
system
r
electrons )
of
,
compounds
these
electron-rich
are
and
therefore
undergo
can
electrophilic
attack
to
electron
alkenes
ring
which
and
reactive
very
are
,
benzene
makes
system
stable
so
,
readily
it
is
undergo
resistant
addition
to
addition
reactions
reactions
:
has
benzene
ring (
electron
conditions
right
,
-
group
:
would
der
of
have
energetically
dispersion
Waals
the
to
non
-
polar
break
feasible
forces
hydrocarbon
many
attraction
of
part
hydrogen
in
the
bonds
between
arene
between
the
functional
the
water
molecules
group
and
means
molecules
to
has
that
be
a
these
boiling
point
compounds
SOLUBLE IN
WATER
of
are
often
which
,
80°C
insoluble
does
not
in
water
happen
as
HALOGENOARENES
✗
these
'
they
'
Properties
chemical
-
the
-
as
molecule
ring
halides
also
take
electrophilic
to
prone
part
SUBSTITUTION
in
and
bromo benzene
,
expect
the
,
lodobenzene
boiling
point
halogenoarenes
arenes
molecules
these
benzene
a
functional
group
because
attack
the
of
system
r
delocalised
of
electrons
REACTIONS
:
,
-
to
liquid
all
are
increase
at
with
attached
halogen
of the
size
as
temperature
room
oily
an
texture
because
increases
number
the
of
,
electrons
the
within
increases
other
like
-
are
also
can
might
you
bonded
HALOGEN
a
halogenoarene
halogens
chlorobenzene
contain
:
Physical Properties
-
that
aryl
as
compounds
these
-
known
also
are
compounds
aromatic
are
halogen oarene
to the
relative
large
are
to
molecules
insoluble
are
break
s i ze
between
bonds
hydrogen
the
molecules
water
of
because
water
in
of
and
,
the
water
NON
the
-
with
as
the
molecules
hydrocarbon
POLAR
arenes
it
so
,
does
it
is
not
part
the
of
ring
energetically favourable
not
for
the
happen
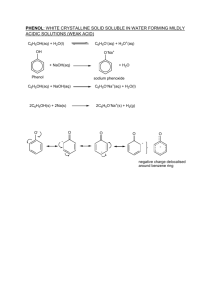
PHENOLS
phenols
'
'
chemical
-
-
-
-
Properties
the
this
it
/
-
OH
phenols
group
due
is
to
aromatic
of
INCREASED
an
it
makes
the
compounds
more
with
acidic
DENSITY
the
for
react
containing
a
bonded
HYDROXIDE
to
benzene
a
ring
Of
than
the
hydrogen
reactive
alcohols
in
ring
of
metal
as
it
causing
,
the
-
OH
such
the
as
become
to
group
donates
oxygen
to
be
sodium
one
much
of
its
more
lone
pair
reactive
of
electrons
than
into
the
benzene
system
ring
itself
.
donated
to
form
ALKOXIDE
IONS
:
white
,
-
is
ELECTRON
easier
also
can
a
phenols
is
Properties
phenol
water
type
:
causes
also
Physical
-
another
are
OH
crystalline
group
in
and
solid
it
,
phenols they
,
can
has
a
disinfectant
fmornmnnhymdnrgyggennmbonndns
like
with
.
phenol functional
group
smell
water
molecules
,
and
therefore
to
a
degree
phenol
is
soluble
in
ACYL CHLORIDES
'
chlorides
acyl
( carboxylic )
are
chlorine
-
a
acid
attached
atom
acyl ( hydrocarbon )
an
containing
derivatives
to
C
a
group (
0
wnr nvunuwmrhu
attached
group
to
a
:
C=O
would
what
replacing
have
been
the
-
OH
group
chlorides
acyl
Properties
Chemical
-
they
-
11
chlorides
this
-
reactivity
why
Physical Properties
•
chlorides
acyl
-
and
they
-
-
'
liquids
fuming
are
a
strong
smell
mmmmm
readily
and
take
part
have
why they
with
violently
the
are
such
carboxyl C-
Properties
water
so
cannot
we
say
,
whether
or
not
chloride
functional
also
building
COOH
react
react
reaction
Physical Properties
-
most
amino
of
the
acids
the
react
they
they
would
be
soluble
in
blocks
of
group
proteins and
consists
of
)
.
group
with
bases
to
É
form
of
alcohols
with
amino
acids
to
form
with
-
acid
functional
esters
amines
give
amides
:
amino
have
acids
chiral
are
centres
soluble
and
in
NH ,
salts
amino
-
-
CI
-
R
amino
the
smell
11
C
-
:
acids
-
-
strong
a
which
in
C- NHZ) group
amine
chemical
they
reactions
substitution
in
ACIDS
an
a
and
with
,
chlorine
with
:
react
acids
amino
colourless
reactive
acyl
'
chlorides
'
mmmm
R
AMINO
acid
are
extremely
are
is
'
as
C- Cl
>
:
liquids
fuming
Mmmmm
are
acyl
-
known
also
are
acid )
o
o
group
C- OH
'
carboxylic
a
of
water
exhibit
but
optical
insoluble
isomers
in
organic
( except
for
solvents
glycine ]
group
water
.
atom
any
,
is
water
substituted
vapour
by
in
other
the
species
air
AMINES
'
'
amines
classification
Primary
-
tertiary
-
Chemical
Properties
due
-
the
to
they
-
the
-
-
lone
the
-
pair
smaller
they
often
C-
N
→
NH )
the
N
the
>
of
N
the
-
)
N
the
of the
the
of
group
amine
amine
amine
group
bonded
is
bonded
is
group
bonded
is
to
three
to
R
group
( and
and
groups (
R
two
to
R
one
one
2
hydrogen
atoms
)
atom )
hydrogen
groups
pair
of
electrons
the
on
nitrogen
COMPOUNDS
BASIC
are
amines
,
:
the
of
amine
soluble
often
are
-
group
:
lone
Physical Properties
'
C
amine
amine
:
Amines C- NH )
≥
amine
the
with
amines
of
secondary
-
-
compounds
are
group
because
water
in
means
i m
VERY
are
amines
have
fishy
a
soluble
smell
water
in
especially
,
that
they
they
form
hydrogen
but
their
solubility
,
as
hydrogen
form
can
bonds
bonds
with
decreases
water
the
as
molecules
non
-
wnr nnnr nvneunvn
the
size
the
of
amines
hydrocarbon
polar
chain
gets
longer
increases
R
B
R
NH
primary
N
R
NH
R
Z
amine
secondary
R
amine
tertiary
amine
AMIDES
'
amides
are
compounds
C- NHZ )
-
an
-
-
Chemical
.
-
^
a
the
amine
carbonyl
amide
Properties
amides
-
amides
the
are
smaller
%
:
R
is
group
C=0
-
-
NHZ
functional
amide
group
group (
C
-
group
)
CONHZ
:
less
are
Physical Properties
-
containing
often
amides
basic
than
amines
,
as
the
lone
pair
of
electrons
the
on
nitrogen
is
delocalised
:
soluble
are
very
in
water
soluble
as
in
they
water
,
can
but
form
their
hydrogen
solubility
bonds
with
decreases
water
as
the
molecules
non
-
polar
hydrocarbon
chain
gets
longer
Nomenclature
Nomenclature
of
of
simple
simple
aliphatic
aromatic
organic
organic
molecules
molecules
Terminology
ELECTROPHILIC
act
Reaction
in
Mechanisms
SUBSTITUTION
that
species
can
used
as
Electrophiles
electron
an
electron
are
-
and
deficient
electron
are
pair
acceptor
loving
species
that
reactions
are
atom
the
involve
replacement
.
one
of
another
by
atoms
of
group
or
7
~
Electrophilic
Substitution
Reactions
reactions
which
in
atom
an
electrophile after
Example
-
'
a
group
by
attack
initial
atoms
of
the
replaced
are
defiant
electron
by
an
species
:
bromine
benzene
1-
bromine
acts
as
an
hydrogen
atom
is
substituted
the
or
"
"
" ro "
" "3 "
' " "" "
and
electrophile
by
bromo benzene
s
bromine
a
the
attacks
electron
to
atom
hydrogen
t
-
rich
bromide
benzene
bromo benzene
form
ring
and
hydrogen
bromide
Br
anhydrous
Brz
1-
AIBR }
catalyst
-I→EoooBTE %F↳
t
acÉÉ=EaoEoa_-→---o
HBR
↳ electrophile
'
benzene
undergoes
substitution
reactions
rather
than
addition
because
reactions
of
combine
two
to
or
give
more
yyggy.mg/g, m,na,am,.m
single
a
involves
molecules
product only
stability
of addition
reverse
when
the
the
µ,
benzene
the
of
reactions
loss
of
ang ,
,
small
a
molecule
ma ,
man ,
Reactions
Example
↳ ""
:
" "" ° "
""
"
" "
H
H
Pd / C
/
"
"" "" "
H
"
°"
H
:
"
"° "
NaOH
""
"
"" "
"
H
µ
H
.
H
C
C
,
H
alkene
H
H
µ
heat
H
H
alkyl
hadide
"
"" "
H
H
É_⇐-EEBE¥ J•
C.CH#-H----q-Eo9Trq--F%9CCc--C
+
H
H
Example
ring
µ
alkene
\
H
°
,
shape
AROMATIC
COMPOUNDS
consists
-
→
shape
-
orbital
this
-
and
benzene
aromatic
&
Aromatic
often
that
means
carbon
each
atom
hybridise d
sp2
contain
benzene
in
carbons
as
and
each
carbon
'
the
remaining
-
this
extensive
entire
and
@•BBB
atom
p
the
in
orbital
sideways
other
delocahsation
double
and single
delocalised
two
of their
mixed
have
orbitals
p
with
an
S
ring
Bag
laterally
overlaps
overlap
of
three
p
orbital
p
sp
H
≥
C=c
•
q7'0•@%
,
H
spzhybndised
0
bonds
with
orbit -1s
one
Ls
••
forms
has
results
p
orbitals
in
the
spa
the
using
of
orbitals
carbon
neighbouring
electron
being
atoms
delocclised
and
to
form
able
to
a
r
bond
freely
spread
over
the
ring
benzene
the
are
]
Mq
py
-
double
electrons
odours
compounds
aromatic
other
Bgb
ˢ
'
which
in
.
¥¥
'
alternating
from
bonds
systems
r
pleasant
have
arise
compounds
compounds
aromatic
other
they
as
Molecules
conjugated
with
rings
more
or
one
of
are
Benzene
of
Aromatic
Benzene $
of
bond
of
character
aromatic
electrons
compounds
means
that
are
all
regular and
the
carbon
planar
-
carbon
compounds
bonds
with
in
bond
these
angles
compounds
of
are
120°
identical
and
have
both
single
and
·
bonds
the
OF
STRUCTURE
·
·
·
these
bands
there
would
also
no
instead
that
double
planer
a
gives
ef
⑭
bands
no
oft
th
6
<
anongo
single bonds
the
and cameras
break
it
of
any
down
for
through
hydrogen
in
any
and
localised (in
three
all
on e
bond
·
n
fe
aBoots
⑭
of
position)
lengths
ooooo
oo
a
evidence
is
relocalised
the
ring
structure
benzene
of
one
around
would be
position)
the
double
mng
by
bonds,
the
overlapping
six
the
pi(/)
it
electrons
orbitals.
equal
structur
·
⑱b
length
same
relocalised (not
were
be
the
BENZENF-DELOCALISATION
theory suggested
the
It
being
all
normal
atoms
can
sank
not
oo:
a
possibility
an
electrophilic addition.
would
·
affect
However, substitution
decolisation
mg
·
·
m
avons
*
aBoot
⑭
anema
delocalised
orbital
Pi
system
-----
that
make
up
Properties
Enantiomers
of
Stereoisomers:
there
two
are
the
have
that
molecules
types
of
same
structural
formulae
but
have
the
atoms
arranged
differently
in
space
stereoisomerism:
geometrical (cs/trans)
optical
Optical
Isomerism
carbon
atom
compounds
with
a
ENANTIOMERS
has
a
your
different
chiral
centre
atoms
or
(chiral
group
of
molecules)
atoms
attatched
exist
as
as
two
it
is
called
OPTICAL
a
CHIRAL
ISOMERS
CARBON
which
a re
or
CHIRAL
also
CENTRE
known
as
H
groups
µ
centre
chiral
[
§
4
Group
↓
←
OH
I
2
group
Br
t
this
chiral
centre
( mirror
images
1
group
gives
rise
which
are
two
to
non
enantiomers
imposable )
super
-
:
:
H
I
H
I
Hsc 1111C
1
°"
Ask
C
ICH }
Br
I
Br
"/
B.
Ho
l
I
enantiomer
1
2
enantiomer I
'
-
the
their
enantiomers
physical
hence
one
of
whereas
the
are
and
non
-
chemical
isomers
the
optical
the
other
are
imposable
super
properties
called
will
will
isomer
it
but
the
in
plane
of
polarised
"
differ
ability
their
in
polarised
in
the
to
clockwise
rotate
~
~
plane
polarizer
sht
direction
direction
¥
,
on
other
.:*÷:%¥ ;•÷÷i¥r
>
J
L
each
AM
^
4
they
anticlockwise
the
'
•
of
ISOMERS
rotate
rotate
images
IDENTICAL
are
OPTICAL
isomers
mirror
polarized
light
→%k
=-B_→rEoroSAgpÑE¥
1←oc5EEÉE_gz→E=-←-
when
light
unpolansed
becomes
polarised
light
as
the
is
passed
waves
will
through
vibrate
a
in
polanser
one
,
the
plane
only
plane polarised light
-
Biological
enantiomers
-
-
they
'
are
exact
CHIRAL
let's
•
there
similarly
speed
which
is
binds
by
reactions
binding
specific
to
chiral
a
enzyme
the
ACTIVITY
binding
(
site
substrates
also
called
image
mirror
,
and
Compounds
if
,
mixture
of
Racemic
of
and
site )
active
will
bind
only
that
molecules
have
the
this
enantiomer
will
not
bind
nearly
as
well
if
at
all
mixtures
be
there
case
the
rotated
is
racemic
80%
mixture
:
of
20%
enantiomer
the
is
a
light
enantiomer
rotates
plane
of
enantiomers
plane
polarised
mixture
of
the
the
polarised
reaction
light
will
reversed
mixture
is
be
the
rotated
which
rotates
the
,
in
which
there
are
80%
of
said
to
be
OPTICALLY
which
,
reaction
plane
equal
rotates
the
mixture
is
still
OPTICALLY
plane
ANTICLOCKWISE
CLOCKWISE
amounts
of
ACTIVE
anticlockwise
,
enantiomer
CLOCKWISE and
light
ANTICLOCKWISE
so
a re
the
CLOCKWISE
,
and
polarised
of
which
enantiomer
the
of
,
the
of
20%
is
each
that
is
percentages
will
there
,
plane
the
effect
the
this
in
rotates
net
light
solution
a
in
uneven
an
polarised
'
a
BIOLOGICAL
their
of
chemical
up
have
they
as
Active
that
suppose
the
-
that
terms
in
Activity
enantiomer
-
other
owngmennanntnmmner
Optically
Optical
each
specific
-
enantiomers
of
shape
if
,
from
PROTEINS
target
very
same
therefore
'
differ
also
are
enzymes
activity
enantiomers
present
in
the
solution
ACTIVE
but
now
the
plane
of
the
planeorpd-nsedhshtmw mhn otch-ng.E /-
•
a
feet
-
'
-
mixture
racemix
these
the
with
isomers
major
other
'
-
these
e.
enantiomer
g.
,
the
rotation
pass
plane
depending
no
are
effect
on
will
that
that
be
the
Light
isomers
and
enantiomers
the
rotates
the
rotates
plane
polarised
which
optical
as
and
non
are
is
that
-
super
one
of
imposable
images
enantiomers
the
of
each
rotate
plane
other
polarised
light
in
a
CLOCKWISE
fashion
therefore
of
Polarised
Plane
ENANTIOMERS
2
ewnauntnonnernnnwlnenngnuennnntnnengnnhhwerns
nnefntent
the
as
On
exsist
the
between
ANTICLOCKWISE
enantiomers
therefore
CENTRE
called
also
enantiomer
the
the
a re
difference
an
in
CHIRAL
a
INACTIVE
Isomers
Optical
Of
molecules
OPTICALLY
is
isomer
observed
said
plane
be
to
polarised
light
the
when
plane
anticlockwise
light
sample
can
be
contains
sample
is
is
used
sample
a
called
is
called
the
R
the
S
enantiomer
enantiomer
active
optically
through
the
clockwise
to
determine
the
of
the
containing
the
,
a
one
plane
RACEMIX
of
identity
two
polarised
of
optical
light
will
an
optical
isomers
be
of
isomer
of
single
a
rotated
either
a
substance
clockwise
MIXTURE
•
¥50
__ -
•Egz§-gp←•§§
.•↳
a.
9
php
%§§
3mg
substance
single
or
anticlockwise
manner
and the
Chirality & Drug Production
'
most
•
-
these
drugs
the
of
drugs
another
that
therefore
can
exsist
difference
crucial
used
are
diseases
treat
to
the
between
differ
which
ENANTIOMERS
as
contain
enantiomers
is
one
chiral
more
each
from
their
in
or
other
centres
biological
potential
ability
their
in
to
and
activity
rotate
polarised
plane
therefore
their
light
effectiveness
as
medicines
-
drug
compounds
should
drug
some
Potential
'
biological
conventional
if
the
E.
'
and
physical
g.
drug
chemical
another
'
enantiomer
one
whereas
-
where
-
one
enantiomer
another
is
of
the
due
to
which
-
this
the
different
results
as
-
it
also
a
in
a
decreases
a
more
in
result
the
potent
,
it
patient
drug
isomers
is
MIXTURE
will
produced
,
in
order
INCREASE
to
THE
DRUG
EFFECTIVENESS
effects
drug
equal
are
pain
amounts
naproxen
the
eases
that
the
of
the
are
patients
in
RACEMIC
a
,
SAME
however
from
suffer
obtained
enantiomers
two
,
be
they
,
may
have
opposite
biological
activities
arthritis
pain
tuberculosis
treat
used
that
side
harmful
very
blindness
causes
drug
a
effective
is
to
treat
effects for
morning
the
unborn
sickness
,
baby
mixtures
activities
the
reduced
side
optical
damage
drug
of
biological
put
are
to
caused
racemic
of
liver
this
example
enantiomers
separating
'
used
of
the
of
enantiomers
the
treat
to
causes
drug
a
one
desired
the
there
,
of
enantiomers
enantiomer
of
another
thalidomide
-
the
of
mixture
used
>
only
harmful
very
make
to
properties
naproxen
one
used
are
racemic
a
have
can
that
way
a
enantiomers
of
reactions
organic
such
in
enantiomer
activity
in
•
prepared
be
side
effects
protects
's
a
in
,
it
is
very
important
to
separate
a
racemic
mixture
into
pure
single
enantiomers
patients
pharmaceutical
dosage
has
enantiomers
product
drug
-
of
by half
better
companies
as
the
therapeutic activity
from
pure
legal
actions
enantiomer
if
is
the
side
more
effects
POTENT
are
too
and
serious
therefore
reduces
production
costs
chiral
-
order
in
the
-
Catalysts
produce single
to
benefits
chiral
using
of
example
for
optical
pure
,
catalysts
isomers
that
are
ruthenium
organometallic
an
,
small
only
catalysts
chiral
,
amounts
catalyst
can
them
of
used
is
in
be
used
needed
are
the
and
production
they
which
naproxen
of
reused
be
can
'
C
"
=
,
CH
stereoselectivity
•
-
due
the
to
the
from
the
disadvantage
'
although
advantages
using
it
E.
routes
only
,
ONE
to
ENANTIOMER
INERT SUPPORTS
on
treat
form
IS
that
so
stereo
the
and
selectively
enantiomer
one
formed
reactants
can
the
over
the
in
produce
single
-
enantiomer
products only
other
reaction
pass
over
them
without
having
to
later
separate
the
.
enzymes
:
that
recently
in
g.
enzymes
promote
reaction
a
of
to
arthritis
that
catalysts
preference
[ used
formed
is
it
be
can
expensive
carried
to
isolate
designing synthetic
them
from
living
organism
out
has
research
into
more
w r r ri n r r r r nvn u rlr n u r n r r r r n n r rlvvvh h h r r n h r r r vh
nir
nw
mw hw mw n m r ~ m m
,
enzymes
to
of
using
of
chiral
that
ensures
enantiomers
the
of
the
place
in
enzymes
one
to
site
fixed
are
therefore
refers
binding
specific
enzymes
product
•
the
:
and
catalyst
biological
excellent
are
,
naproxen
catalyst
only
enzymes
arthritis
COOH
←ocEÉ←EaoEE=-←---E
,
Rahmat
-
of
,
EÉ-EooBTE %↳
Hz
t
COOH
ruthenium
treatment
¥/ 57
3-
i
chiral
the
in
H
H
H
CH
used
is
the
using
to
produce
long
pure
been
enantiomers
in
drug
synthesis
takes
longer
than
enzymes
conventional
synthetic
routes
there
are
,
many
run
enzymes
to
synthesise
drugs
is
a
GREENER PROCESS
as
fewer
steps
are
involved
compared
to
conventional
synthetic
Reaction
'
arches
this
-
-
'
VERY
are
during
chemical
Addition
negative charge
,
a
such
disrupt
however
undergo
arenes
the
reactions
Reactions
due
COMPOUNDS
STABLE
because
is
Arenes
of
series
of
delocahsation
to
SPREAD
is
SUBSTITUTION
as
aromatic
the
REACTIONS
OVER
THE
ring
instead
MOLECULE
delocalised
this
,
the
in
ring
of
confined
being
to
small
a
area
maintained
is
stabilisation
including
reactions
OUT
electrons
IT
of
:
substitution
friedel-crafts
nitration
oxidation
complete
friedel-crafts alkylation
acylation
hydrogenation
SUBSTITUTION
-
'
halogenation
arenes
form
reactions
undergo
are
substitution
halogenoarenes Caryl
the
the
of
reactions
with
substitution
chlorine CCI
,
)
and
reactions
bromine
( Bra )
in
the
presence
anhydrous
of
Alas
or
A / Brs
catalyst
reacts
respectively
to
halides )
chlorine
catalyst
electrophilic
examples
bromine
or
is
required
acts
for
the
as
and
electrophile
an
reaction
to
take
place
OVERALL
,
replaces
due
a
the
to
hydrogen
stability
atom
of
on
benzene
the
benzene
the
ring
structure
REACTION
Brz
+
Brz
AIB.rs
t
>
anhydrous
Sigma
HBR
complex
yi]
'
step
-
1
attack
by
f
:
electrophile
of
aromatic
forming
-
-
formation
breaking
of
Br
C
-
Br
C- c
⊕
+
+
slow
bond
bond (
Br
Brt
bond
carbocation
a
of
r
→
f
'
'
]
H
'
£
Br
HBR
-
t
>
fast
+
r
)
↳
step
-
2
deprotonation
restore
its
substituted
:
of
the
aroma
city
product
carbocation
resulting
in
to
a
two
are
substitution
substitution
'
types
the
into
the
in
substitution
of
that
takes
Reagents
:
place
alkylarenes
for
:
ring
happens
ring
→
>
electron
which
group
-
the
donating
-
"
CH
°
activates
and
2
"
alkyl
4
1-
2C /
CH
,
Alas
t
>
anhydrous
a
positions
]
"
t
ZHCI
-
chloride
aluminium
-
→
if
chlorine
using
bromide
-
aluminium
if
→
chloro
z
↳ ˢᵗˢ
""
benzene
methyl
C'
4- chloro methyl
benzene
bromine
using
-
chlorine
-
or
bromine
Generation
Conditions
:
or
electrophile
of
UV
light
ˢ
:[ 1
2
-
+
/◦
'
:&.it
-1
CHS
Alas
Alas
"
>
fast
2
6
>
s
3
'
_
:
u
temperature
ˢ
Alas
1
H
H
:
°
y
,
a
ÉI
z
t
attack
room
:&.it
>
°
electrophilic
4
-
valets
:
- -
3
s
,
CHS
"
absence
%;
:& ,
CHS
-
6
-
"
ci
OR
>
!&rᵗ
:
it
2
gG
slow
'
S
3
methyl
the
.
-
in
with
methyl
group
other
words
,
chlorine
,
Multiple
-
multiple
is
directing
2,4
are
groups
in
the
substitution
the
new
1
group
into
the
which
that
means
,
Albro
3
S
2
H.BY
t
will
attatch
ring
gives
the
to
incoming
a
next
ring
mixture
of
2-
will
tend
methyl
group
groups
to
into the
go
2
or
4
door
the
to
and
chloro methyl benzene
or
opposite
4- chloro
it
methyl benzene
substitutions
substitutions
occur
when
excess
halogen
is
used
CHS
CHS
Br
+
Brzcexcess )
Br
2,4
CHS
Br
Br
t
>
-
Dibromo
benzene
methyl
:
3
,
position
CHS
3
"
①
>
Br
,
,
-
2
↑
fast
,
,
6
?⃝
?⃝
There
Br
Br
t
+
2,6
-
Dibromo
benzene
Br
methyl
2
,
4,6
-
Tribromo
benzene
methyl
7HBr
positions
on
the
ring
-
assuming
?⃝
Substitution
Reagents
:
methyl
the
into
group
°
-
CHS t
-
chlorine
CH
Brz
,
Br
TH Br
bromine
or
>
absence
catalyst
of
methyl
( bromomethy 1) benzene
benzene
↳
brackets
µ,
the
in
,,
"" " "
Conditions
the
on
:
CHzBr
presence
1-
""
,
if
emphasizes
,
""
,
"
,
,,
"
"
Brz
""
methyl )
benzene
@ ' bromo method )
benzene
+
Brz
C.
1-
Brg
mixture
HBR
>
(dibromomethyl )
benzene
c- nbromomethy 1)
benzene
-
this
-
in
of
:
temp
of
:
of
nitro
a
,
substitution
C- NO )
,
group
reaction
replaces
a
hydrogen
concentrated
nitric
on
the
arene
acid ( HNO )
,
and
conc
sulfuric
acid (11-2504)
-
between
25
-
60°C
NOZ
HNOs
1-
of
( µ,
<
H2O
t
-
nitrobenzene
benzene
Nitration
atom
-
mixture
Conditions
example
reactions
these
Reagents
-
arenes
another
is
""
alkylarenes
electron
group
the
+
2
donating
CH
alkyl
CH
which activates
and
4
positions
HMOs
}
}
N
'
02
t
V02
methyl benzene
"
benzene
,
proportions
CHBrz
Nitration
"
bromine
enough
"
"" "
but
any
>
(bromomethyl
-
use
you
-
Nitro 1- ◦ tune
P
-
you
" "
ring
CH Brat HBR
light
UV
of
name
, ,,
Nitrotolune
of
will
always
products
other
lead
to
a
Friedel-Crafts
'
•
•
friedel-crafts
due
to
to
reactive
'
the
aromatic
like
group
any
ELECTROPHILIC
are
stabilisation
starting
as
materials
in
REACTIONS
SUBSTITUTION
arenes
the
for
they
,
synthesis
UNREACTIVE
often
are
of
other
compounds
organic
,
their
structure
needs
to
be
changed
to
turn
them
into
more
compounds
friedel-crafts
acyl
-
reactions
arenes
use
Reactions
(
reactions
can
be
used
to
substitute
a
hydrogen
atom
the
in
beng
an
for
alkyl
Friedel-Crafts acylation )
other
electrophilic
substitution
reaction
the
,
-
-
-
generating
the
electrophilic
reactions
consist
of
three
steps
:
electrophile
attack
regenerating
Friedel-Crafts
on
the
aromaticity
benzene
of
the
ring
benzene
ring
→
propyl
CHzCHzCH3
FRIEDEL-CRAFTS
1-
ALKYLATION
CHzCHzCHzCl
¥.
t
AIC / 3
propyl
01
FRIEDEL-CRAFTS
ACYLATION
1-
CHNSCHZCOCI
¥+
AIC 13
_
benzene
CHCH }
1- HCl
HCl
group
(
Friedel-Crafts alkylation )
or
an
functional
FRIEDEL-CRAFTS
ALKYLATION
'
this
in
the
-
-
e.
type
benzene
g.
of
step
I
Friedel-Crafts
of
reacted
is
ring
alkylation
with
reaction
Generating
:
reaction
the
in
hydrogen
contains
atoms
substituted
is
with
only
which
are
chain
a
presence
benzene
of
"" ↳
%
C
,
reaction
chain
in
the
Alas
an
of
chloro
into
propane
benzene
[ AKI , ]
t
CHsCHzCHzt
>
ring
catalyst
( CHSCHZCHZCI )
to
electrophile
the
H
CHSCH
chloro alkane
a
>
alkyl
an
,
that
group
and
carbon
arranged
propyl benzene
form
's
Alas
-
H
tert-butyl chloride
chloro
step
2
:
Electrophilic
Attack
CH
+
[
[ Aclu ]
HgCHzCHg
,
CH
CH
,
¥
]
AICI
T.io
when
"
benzene
gonna take
a
&
-
,
step
3
→
¥¥
µ
,
CH
CH
,
CH
due
}
to
alkyl
position
[ Aklu ]
+
¥
1A
the
friedel-crafts
the
>
-
the
e.
g.
benzene
of
ring
that
group
reacted
is
acylation
note
acylation
acyl
an
'
would
the
HCl
t
not
alkyl
benzene
a
be
more
*
+
"
Its
¥
A
◦
→
reaction
an
acyl
reaction
is
alkyl
an
with
>
chloride
an
group
on-nop-it.cn
P→ para
on
group
of
the
is
containing
chloride
acyl
reaction
is
acyl
an
,
in
the
methyl benzene
4
position
due
a
substituted
into
carbonyl
c=0
presence
with
to
,
of
propanoyl
the
-
CHS
an
the
position
on
ring
catalyst
Alas
chloride
group
benzene
to
the
form
benzene
At
↳
butyl benzene
an
acyl
benzene
,
so
makes
the
than
the
reactive
ring
therefore
continue
favoured
group
benzene
nucleophile
bulky
ortho
④
Alcb
+
•"↳
EXTENDED
-
ACYLATION
FRIEDEL-CRAFTS
in
being
,
-
propyl benzene
'
there
group
-
gonna
reactive
,
"
→
for
>
CH , CH
#
,
bond
Restoring aromaticity
]
→
-
the
normal
CH
Ñ"
is
ring
attack
#
POLY ALKYLATION
:
-
intermediate
break
is
carbocation
its
INTERMEDIATE
,
É
)
]
a+¥¥¥a¥:
Ff
-
c '
-
%
H
>
4-
1)
propane
a
.
It
is
more
stronger
alkylation
will
H
INTERMEDIATE
in
1
step
Generating
:
electrophile
the
◦
& %
CHzCHz
^" "
chat
_
-
.
{
It
+
[ Aldi ]
+
,
g.
R
.
chloride
propanoyl
↳
-
donates
chlorine
1
lone
+
→
2
CH
Attack
Electrophilic
:
pair
Aluminium
to
'
skeletal formulae
D:
0
}
CH
Alas
in
C
-
bond breaks
C'
¢
CH
CH
,
[A
"'
"
]
M
-
>
acyhum
Step
CH
-
CHICA
Restoring
C
H
[ Alay ]
t
f
CHICA
=
Ct
one
to
of
, ,
its
it
,
gonna
pull
e-
being
towards
more
electro
-
itself
take
gonna
lone
pairs
another
form
carbon
]
no
≤
r
bond
?
0
:
is
atom
longer
e-
detkent
:B
}
t
HCl
nugtnnxndnhnd by oxidising
agents
>
1-
AKI
benzene
ring
carbon
with
A / Cly
=
%[
' on
t¥
→
-
"
-
""
CH }
_
,
double bond g
-
carbon
Alai
↓
Aromaticity
CH
n
⑥
-
,
O
:
case :-O
,
than
-
V
C
acyhum
:
-
/
' on
CH
3
a
,
oxygen
CHS
+
H
At Cb
to
-
?¥¥%¥ᵈ
>
,
p
]
due
Cl
"
I/+
0
+
-
+
↓/
_
ÉI
negative
step
Alas
.
-
equilibrium
T
O
-1-1--4 >
acetophenone
charge
1-
}
0
C
]
=
/
0
CHZCH ]
OXIDATION
COMPLETE
-
-
normally
however
the
,
-
alkanes
,
the
alkyl
with
dilute
are
presence
side
chains
-
alkyl
in
in
ring
OXIDISED
are
arches
alkyl
affect
arenes
to
as
carboxylic
manganate
potassium
the
acids
properties
when
g.
the
complete
oxidation
ethyl benzene
of
forms
CHzCHz
the
refluxed
CHZCOOH
-
t
3
heat
[0]
t
>
Hao
-
with
'
the
benzoic
benzene
hydrogenation
benzene
is
side
alkaline
-
chain
potassium
manganate
( V11 )
of
heated
benzene
with
is
an
addition
hydrogen
gas
1-
3h2
and
then
nickel
or
platinum
catalyst
/
benzene
heat
ni
or
Pt
>
Cyclohexane
to
form
Conditions
:
potassium manganate (V11)
acidified
acid
reaction
a
&
reflux
alkaline
HYDROGENATION
'
alkyl
Reagents
benzoic acid
-
ethyl
of
( V11 ) ( kMnO4 )
acid (11-2504)
sulfuric
e.
benzene
the
of
such
cyclohexane
dilute sulfuric
acid ( Hzsoy )
and
then
acidified
-
the
reaction
same
CH
,
CH
occurs
when
ethyl benzene
undergoes hydrogenation
to
CHZCH ]
}
r
,
heat
1-
3Hz
Ni
or
Pt
>
ethyl
cyclohexane
form
cyclo ethyl benzene
Arenes Electrophilic
substitution
:
electrophilic
the
-
I
generation
-
substitution
2-
electrophilic
attack
3-
regenerating
aromaticity
MECHANISM
-
ELECTROPHILIC
OF
and
halogenation
the
a
arenes
in
consists
three
of
steps
:
electrophile
an
of
reaction
nitration
hydrogen
SUBSTITUTION
atom
replaced
is
both
are
arenes
of
by
a
examples
halogen
electrophilic
of
atom
or
a
nitro
reactions
substitution
C- NO )
,
group
By
①
OVERALL
REACTION
chaos
" ""
"
"
AlBr3
Brz
+
anhydrous
HNO
t
HNO
REACTION
nitration
of
arenas
step
-
t
HBR
]
t
,
Hasa
]
Addition
Arenes
of
Nitration
Overall
Step
or
Proton
f
stable
-
due
to
losing
-
low
energy
molecule
( stable ]
to
with
high
End
↓
{
when
E
stable
'
to
-
energy ( unstable )
1-*
unstable
e¥
E
H
base
generate
is
to
electrophile
-
S
O
-
H
-
"
◦
H
electrophile
↓↑
's
"
N
_
will
take
away H and regenerating
neutron
cloud
that
be
going
to
to
react
used
0
0
from
unstable
-
Anos
11
O
U
-
with
benzene
( NG )
"
A
Hz
c-
+
N
H2O
t
11g
→
any
:B
help
O
+
aromatic ' 'T
goes
"
,
benzene
its
it
"
jji t
electron
pxiii
U
arches
of
Hzsoy
purpose of
Reaction
"
gang from
H2O
]
Halogenation
first
①
→
OVERALL
[
>
on e
p ¥yg→
3
g the
due
to
can
Hzo
be
being
used
:
generated
in
production
of
electrophile
g;→B¥¥◦
0
¥
"
"
N④
#
H
Nitrobenzene
hydrogen atom
replaced with
is
electrophile
I
generated
Selectrophile
Step 1: generating
electrophile
the
concent
-> oative
BrAIBr
Br
Brt
>
[ABry]
+
-
↳ electrophile
-
halogen
with
halogen
carrier, which
halogen
the
molecules
halogen
carrier
halogen
atoms
by
form
donating
into
dative
a
lone
a
empty
an
covalent
pair
band
orbital
3p
of
pair
a
this
disrupts
only
four
over
from
electrophile
the
to
electrons
of
the
to
the
the
form
aromaticity
and
te-
five carbon
there
in
is
ring
covalent
the
a
from
of
one
halogen
carrier
ring
HNOy
+
the
~
electrophile,
-
the
C-A
into
aromaticity
band
the
is
so
benzene
restored
that
a
by
is
donated
bond
the
once
-
out
an
bond
bonding
on
NO
electrophilic
with
the
as
It
there
are
now
a
pair
electrophile
spread
to
system
clearage
this
of
bond
the
go
the
C-A
the
four
the
a
the
the
benzene
ring
and
H.SOn
refluxed
is
aromaticity
and
carbon
ring
donated
is
to
the
bond
covalent
te-
five
there
in
the
is
a
ring
as
there
are
positive charge
now
spread
atoms
NO2
·If
aromaticity
band
benzene
carry
"E
from
form
disrupts
this
will
23-60 (
wont
electrons
of
it
aromaticityis lost andtchargemoms
fiftiesshorinwgron
in
benzene
attack
only
electrons
at
sulfuric and (H2SOu
HNOy
of
with
it
generated,
been
the
mixture
arena
by reading
concentrated
on
H, Ot
+
on
generated
has
attack
nitrating
Aronium
is
and
electrophile
the
-
positive charge
heterolytic
NO, +2HsOn
,
acid (HNOz)
nitric
conc
atoms
cons,
2H, sO
&
over
To
electrophile
the
-> electrophile
electrophilic
benzene
a
the
the
Step 1: generating
↓
"
Fie
with
electrons
of
aromaticityis lost andtochargemoms
-
generates
electrophile
the
-
reacted
is
is
is
so
a
restored
that
bonding
by
it
heterolytic
band
system
electrons
clearage
in
this
of
bond
the
go
into
ADDITION REACTIONS
-
the
delocahsation
substitution
'
in
addition
over
reactions
substitution
other
as
one
:
arced
two
elements
heterolysis
Q
element
#
in
arenes
in
is
the
reason
main
why
by
HETEROLYTIC
CLEAVAGE
of
C
the
-
bond
H
can
break
which
way
a
in
one
element
retains
has
lost
and
,
the
pt
other
+
has
①
reactions
.
the
bonding
pair
=
gained
an
electron
,
positively
and
negatively
→
charged
ions
are
formed
MF
_
H
H
completelylost@E.g
ÉÑexane
addition
predominantly undergo
arenes
-
F¥ :
-
substitution )
loses
p
-
the
between
bonds
the
:
aromatic
reactions
>
Hetero lytic cleavage
-
called
electrons ( also
of
reactions
ARFNGS
Of
the
hydrogenation
aromaticity
is
not
restored
and
in
some
formed
cases
is
energetically
less
stable
than
benzene
and
Arenus:Location
·
thalogens
crenes
halogenation
in
in
aromatic
the
side
AROMATIC
of
alkylarenes
RING
the
in
aromatic
#
I
anhydrous
less reactive towards
it
s nucleophilic
band
carbon-halogen
due
is
the
to
carbon-halogen
any/halides
in
partial
overlap
of
band,
#"Ttherefore
the
-
16Br
substitution:
are
this
+
bromobenzene
benzene
-
occur:
used
is
Br
.
catalyst,
Brc
+
will
ring
hcloserand onlydoesit
↑
the
has
lone
is
on
pairs
partial
a
scop
STRONGER
IS
the
sp>
is
the
band
lorbin
halogen
&
6%"
a
&
atom
Breaking
(ii)
spc
is
hybridised
bond
ofC-X
conjugation between
lone
requiring
pair
of
this
the
benzene
ring
foeo"
0
O
A
(b)
results
greater
electrons
in
"a
>
(a)
carbon
system
A
3
&
(i)
#
1
"
o
=
->
the
with
"IT
1
(583,
A
-
a
6
atom
characein
M
+
reaction
chain
halogen
-
ofHalogenation
reacton/halogenation
ring
IN THE
halogenation
3
halides
aryl
substitution
occur:
can
the
↓ ALOCONATION
S
-
more
in
a
character
and
shorter
bond
length
requiring
high
energy.
energy
on
halogen
atoms
and
pielections
of
aromatic
ring
->
&.overlap ofonep-orbital but
resulting
in
resonance
of
double-
in
sigma
bond
bond
character
for
C-x
bond
co
(iii)
self
conization
electrons
of
ofaromatic
anyl
ring
halide
block
will
give
backside
phenylcation
attack
of
which
will
nucleophile. Hence,
not
SN"
be
stabilized
mechanism
is
by
ruled
resonance.
out
Hence,
sit
attack
is
not
possible.
Pi
NOTES
HYBRIDISPTION
SP
DETERMING
ON
OF
A
COMPOUND
is?"
~H.C-
"s**-c-co↓
find
first
you
the
stent
no.
no.-fairs lovee gooracomsbordert
Stene
stenc
If
↑
+
C
1s.....
sp3
3
=
Spe
Ou
sp
OS
2-
=
3
8"
Hs?
#2CSC-cspe
to
4
=
sp2
sp3
4
=
Cs
S
-
3
=
C,
-
=
1
no.
'E_CHs
"-
-is_pitc-"I
3.
sp2
4
C,=
sp3
-asi
I
Yte
No 4
=
=
Ca
2
Co
HALOGCNATION
·
halogenation
of
alkyl
-
arenes
halogen
this
-
*
THE
IN
is
a
side
passed
is
CHAIN
SIDE
the
in
chain
If
C12
boil
Or
>
light
*
chloromethyl
in
the
presence
ultraviolet (UV)
of
light
halogen
used,
is
all
&
Cl
benzene
atoms
hydrogen
atoms
*
NO
alkylarence
*cC
↓
excess
halogen
when:
occur
substitution
methylbenzene
·
will
boiling
into
radical
free
2
x
OCCUR
UNDER
the
CCly
ball
Cc (excess
SUBSTHOTION
on
IN
or
light
BENIENT
THESE
#
RINGS
CONDITIONS
↓
3HCl
alkyl
side-chain
will
be
substituted
by
the
Arenus:
Directing
substituents
·
takes
undergo
readily
arenes
that
already
are
ELECTRON-WITHDRANING
substituents
the
on
ELECTRON-WITHDRAWING
these
e.g.
the
on
deactivates
arenes
nitro
the
the
DONATING
to
benzene
(ii)
NO,
one
with
atoms
substitution
the
where
affect
can
hydrogen
their
of
another
the
of
species
hydrogen
attack
bromination
products
of
being
is
atom
the
on
substitution
to
reactions
arene
positions
of
it
electron-donating
or
system
direct
in
the
electron-withdrawing
an
the
bromine
and
electrophile
the
groups
benzene
to
LESS
t
attack
REACTIVE:
the
<N '*
3
and /
or
5
positions
-
directed
be
will
making
electrophile
incoming
group:
ring
to
the
and /
3
5
positions
electron
density
or
5-chloronitrobenzene
effect
No,
(-1)
-
dectivola
than
electronegative
more
and
electrophiles
nitrobenzene,
inductive
the
from
3-chloromtrobenzene
are
by
ring
by
nitrobenzene
in
withdrawing
electron
movemelectiononsity
group
direct
undergo
alkyl
groups
benzene,
in
it
will
withdrew
ring
decreases
#
avenes
to
be
One
of
GROUPS
either
can
deactivate
groups
the
B
said
are
groups
SUBSTITUONTS
upon
(i)
present
substitution
substituents
of
place
these
the
electrophilic
Effects
from
the
R
If
the
density
charge
N
>
I
#
at
ortho
and
0
0.,-0②"
C
>
2
.
L
*
A
8)
p
electron
positions
meta
-
electron
position
para
rich
deficient
of
the
relative
ring
N108
&
c
J
C
>
a
to
v
meta
O
I
para
->
=>
ortho
reta
·ELECTRON-DONATING
-these
-
e.g.
the
SUBSTITUENTS
umumumatron density
Smuum
activate
by electrophiles
groups
methyl
upon
the
group
in
attack
methylbenzene
bromination
products
are
is
an
methylbenzene,
of
into
and
direct
electron-donating
the
bromine
2-chloromethylbenzene
and
the
it
the
system
incoming
of
the
electrophile
benzene
to
attack
the
2.4
and /or
group
electrophile
will
be
directed
4-chloromethylbenzene
to
the
2
andlar
A
making
ring
4
position
6
MORE
positions
REACTIVE
Production
·
halogenoarenes:
a re
they
SUBSTITUTION
·
chlonne
prepared
be
can
BENZENE
OF
arenas
TO
bonded
are
SUBSTAUTION
from
FORM
bubbled
is
gas
which
halogen
to
REACTIONS
of
with
avenus
benzene
into
at
and
temperature
room
oo
·"sonprience ↑
the
Ally
the
·
chlorine
or
bromine
the
in
presence
of
anhydrous
catalyst
HALOGENOARENES
in
the
presence
anhydrous
an
of
All catalyst
to
form
chlorobenzene
Groom tempe
of
Generating
Halogenoarenes
of
atoms
electrophile:
catalyst
is
also
called hydrogen
AC,
#
and
carrier
required
is
to
generate
the
electrophile (c)")
CAICk]"
>C1++
1:
Stage
this
attacks
electrophile
·
the
benzene
electron-rich
ring
the
in
first
stage of the
reaction
which
disrupts
the
delocalised
it
system
in
the
ring
CI
A
Dt
&
benzene
stage
A
electrophile
aromaticity
is
lost
2:
to
restore
·
when
this
the
aromatic
happens,
the
stabilisation,
delocalised
a
it
hydrogen
system
in
atom
the
is
ring
removed
in
the
second
stage
AlBry
catalyst
of
the
electrophilic
substitution
restored
is
Cl
Cl
It
CA1C1y]-
t
↓
>
&
Chlorobenzene
the
same
reaction
occurs
with
benzene
and bromine
HCl
+AICls
aromaticity
in
the
is
restored
presence
of
an
to
form
bromobenzene
reaction
to
form
chlorobenzene
S
SUBSTITUTION
the
·
this
electrophilic
this
the
·
the
TO
with
methylbenzene
of
methyl
group
HALOGGNOARENGS
FORM
halogens
(which
alkyl
an
is
results
in
the
group)
formation
methylbenzene
in
halogenoarenes
multiple
of
is
as
ELECTRON-DONATING
products
and
pushes
benzene
makes
the
methyl
group
said
is
substitution
to
~
same
activates
towards
alkyl
the
2
the
as
as
and
chlorine
with
substitution
a
result,
anhydrous
2
and
4
positions
catalyst, therefore,
AICIs
into
the
ACTIVATED
are
gives
2
-chloromethylbenzene
benzene
mechanism
of
and y
CH3
↓
the
positions
CH3
x
I,
anoris
2C,
/
2
methylbenzene
-
#
"
A
chloromethylbenzene
4-chloromethy
2HC1
+
(
benzene
In
density
reactions
substitution
group
cltz
Ix
electrophilic
2.3-directing and
be
the
is
which
REACTING
methylbenzene
of
mechanism
reaction
MORE
ring
electron donating
·
electron
ring
electrophilic
the
substitution
because
is
benzene
·
OFMETHYLBENING
the
presence
of
substitution
clarine,
excess
the
6th
also
will
position
CH3
Halogenation (1)
occur
CH3
CH3
CI
AKCls
2010 (g)
+
Reagent:C1,
on
A
&
S
2HKI
(g)
Cl
2-chlcromethyl
conditions:
AC's / feels
anhydrous
catalyst
room
4-chlcromethyl benzene
temperature
C11s
(11)
Halogenation
at
benzene
CH3
CH3
Br
Reagent:Bre
2Br2CK
+
(1)
feBry
↓
Br
conditions:
anhydrous
&
I
>
2-bromomethyl
catalyst
feBry
at
room
benzene
↑)
bronomethylbenzene
temperature
MECHANISM
generation
I:
electrophile: c"
of
CH3
AICl,
>
CH3
i:
"
electrophilic
slow,
attack
a
oi"
CH3
I
⑱
·
crepe
fast
&
ic
AICly
>
-
*Cl
21
H-
CT:
ACl,
2HBr
and
4-chloromethylbenzene,
called
also
&
/ alkyl
baloalkanes
-
halocrenes/anyl
Ifference
halides
halides
Reactivity
in
of
Halogenolkanes and Halogenoarenes
· halogenoarenes
the
·
UGRY
are
difference
reactivity
in
compared
UNREACTING
because
is
of
halogenoalkanes
to
the
bond
carbon-halogen
strengths
↓ ALOGGNOALKANGS
the
nucleophile,
·a
overall,
such
bond
covalent
·a
·
halogenoalkane
chloroethane
formed
is
halogen
the
hydroxide
as
part
COH-S, on,
that
between
by
replaced
is
take
can
the
the
in
will
attack
carbon
atom
,phile
=
- - >
T
the
substitution
slightly
reactions
carbon
positive
nucleophile
which
"-"-oN
+
and
the
atom
causes
the
band
carbon-halogen
break
to
nucleophile
,
I
-"c-"
is is
nucleophilic
C
is
chloroethane
ethanol
HALOGCNOARONGS
such
halogenoarenes,
under
only
extremely
this
because
is
one
of
this
causes
by
a
lone
the
pairs
of
such
nucleophile
electrons
is
on
bond
carbon-chlorne
<1:
readily undergo nucleophilic
not
bond
carbon-chlorine
the
the
do
conditions,
harsh
replaced
gets
chlorobenzene
·
chlorobenzene,
as
to
such
very
the
temperature
as
as
have
and
a
:ci:
will
COH-
cannot
interact
partial
pressure
a
be
will
atmospheres,
200
the
chlorine
in
embroken
the
bond
x
system
character,
:Ci
~
the
of
which
ring
STRENGTHENS
band
the
↑ c,
<i+
t
>
-
<
of
on.
with
double
reactions
and
2000
of
hydroxide
a
strong
chlorine
substitution
c
>
>
<
I
>
-
>
C
<
>
2
j
that
electrons
·
the
this
·
unreactivity
causes
additional
halogenocrones
·
It
of
will
halogenoaremes
stabilisation
can
of
the
therefore
system
be
and
explained by
the
strengthens
the
bond
shared
delocalisation
are
between
by
two
three
of
a
or
not
more
lone
carbon-halogen band,
confined
atoms,
to
but
the
carbon-halogen
band
in
halogenocrones,
which
DECREASCS
--
atoms
pair
which
on
the
affects
undergo
to break
gets harder
c u rc m e v l
a
are
REACTIVITY
halogen
the
over
reactions
the
that
benzene
Reaction
chlorides
acyl
carbonyl
the
·
It
the
carbon
therefore
is
carbon
REACTIVE
are
chlonne
bond
acyl
has
a
-
group
positive charge
partial
a
functional
cool
comes
of
chloride,
hydrogen
HCI
was
formed
carbonyl
group
and
alcohols
chlorides
white
-al
R
with
and
breaks
-
·
and
with
Chlorides
Acyl
with
nocleophilic attack o
sssepumunumminenratum
O
Reactions
compounds
organic
electron-deficient
is
Alcohol
of
phenolo
alcohols/phenols,
+
by
esters
nucleophilic
substitution
the-Cgroup asa
R-"goBoom
R.*_0.-H.
-
a
esters
chlorides
acyl
from
chlorides
acyl
acylablonde
Reaction
withAlcohols
acyl chloride
+
It
C
alcohols
"d-c+
A
chlonde
go
than
REACTIVE (so
to
acids
carboxylic
COMPLETION
produces
it
(so
more
the
ester
the
of
because:
effective
more
is
faster)
ester
produced
is
->
O
ethanoyl
more
are
reactions
e.g.
It
rather
R. C-R"
(1
B
I
1""R
forming
+
a
vigorous
reasons
ester
Y-"d-o-1
↓
+
Hlegs (in
the
form
id
-
of
white
fomes]
O
b- "
b
-
eth anal
ethyl
a
ethanoate
↓
HCl
THC1
with
& eactions
chloride
acyl
reacts
acyl
between
16
base
carbon
with
chloride
acyl
chloride
and
an
↓
ethanoyl
it
is
better
needed
to
can
to
the
form
a
con
>esters
the
than
nucleophile
original
at
phenol
temperature, although
room
and
molecule
will
be
able
to
attack
1o
*T
O
11
p-c-
N9016
-
nect
↑ henyl
more
to
form
readily
S
modify
a
fast
as
the
one
the
phenal
phenoxide
0-1T
first
on
to
which
make
is
a
reaction
the
better
faster
nucleophile
phenol;
than
now,
occur.
"T
as
ethanoate
~better
herol
isn't
+ NaCI
C
A
phencl
chlonde
reaction
the
alcohol
↓
C
is
phenol
↑ henoxide
hect
better
a
"P_alt
sometimes
·
a
is
can
deproporate
>
base
carbon
carbonyl
phenol
>
phenols
+
phenoxide
the
·
the
phenols
than
~
phenoxide
con
nucleophile
phenol
the
nucleophilic
attack
on
the
carbonyl
Production
·
Phenols:
phenylamine
·
PRODUCTION
·
phenols
heat
This
the
dilute
keep
1:
3
of
an
of
group
which
is
attatched
to
a
benzene
ring
phenols
and (to
below
form
10
under
the
reaction
following
conditions:
HN0e)
CCstep 1)
Steps:
ANO,
forming
HNOz
is
unstable
so
while
keeping
NaNOz
forming
that
the
it
needs
temperature
HCl
+
nitrate
sodum
2:
presence
(HNO2)
phenylamines
from
temp
involves
ad (Hall
Step
the
(step 3)
reaction
Step
prepared
with
to
acid
by
PHENOL
be
NaNOz
ice
nitrous
+
OF
can
characterised
compounds
organic
Phenol
of
hydrochlone
to
below
be
prepared
10C
nitrous
the
diazonium
test-tube
by reacting
sodium
nitrate
(NaNOz)
and
ice
using
>HNO2
ce
ac
(i.e. H+)
a
in
NaCI
+
sodium
add
chloride
salt
unstable
-
Plazonium salt
L
-
+fWCOSCoro OfelC
Niz
phenylamine
Step
3:
further
NCF
+2H, 8
water
benzene @lazonium
chloude
6)
warming
NN
benzenedlazonium
N
below,
CI
chloude
t
Old
#2 G
heat
3)
azo
NN
group
-
OH
HCI
+
fil
a
phena
azo
sype
hydrochione
c,d
dilute
hydrochlori
Reactions
phenols
chemical
in
of
reactions
both
as
electron-rich
the
benzene
ring
and
the
polar-of
group
can
participate
reactions
include:
phenols
of
bases
with
-
with
reactive
-
with
diazonium
-
types
many
reactions
of the
some
undergo
can
Phenol
of
metals
salts
nitration
bromination
& EACTIONS
OF
THE
the-Ott
· It
Reactions
-phenols
group
therefore
can
with
has
phenols
in
act
as
PHENOLS
IN
and
ACID
an
acidic
slightly
a
character
take
part
acid-base
in
reactions
bases:
slightly
only
are
-however, they
GROUP
OH
-
dissolve
do
~
in
acid
0H
soluble
in
alkaline
water
solutions
due
to
undergo
and
acid-base
benzene
reactions
ring
bases
with
to
form
a
soluble
&
base
f
large non-polar
the
NaOlt
8"Nat
>
-
H28
sodiom
hydroxide
Phenol
-phenol
reacts
this
reaction,
-
In
Sodium
water
↑henoxide
with
the
sodium
hydrogen
hydroxide
con
has
solution
been
removed
to
give
by
the
a
colourless
strongly
solution
basic
containing
hydroxide
on
sodium
in
the
phenoxide
sodium
hydroxide
solution
salt
and
water
with
& eaction
matter
this
is
reactive
phenols
also
react
acid-base
an
phenol
-there
the
is
diazonium
solution
this
the
after
these
reaction
are
-
Step
compounds
will
and
ice
occured,
a
which
in
containing
hydroxide (Noot),
sodium
cold
of
metal
added
is
off
benzene
two
solution
a
solution
rings
NI"
an
diazonumson
yellow-orange
or
are
is
group
sodium
of
added
precipitate
by
linked
=F-O-Na"
NaOHt
+
odium
phenol
Step
phenoxide
on
of
a
phenoxide
sodium
the
to
obtained
is
and
compound
formed
is
nitrogen bridge
420
+
phenoxide
2
#v=N
benzene
diazonium
+
con
Iy
phenoxide
F-N
or
·
Reactions
phenols
this
-
this
is
more
readily
because
increases
the-Ol
aromatic
of
the
group
one
electron
in
ring
reacts
of
phenols
=
N
=
-
01
con
aza
-
piece
1
↑
F0d
-
small
a
phenoxide
sodium
contain
and
motten,
is
given
is
gas
compounds
reactive
in
has
hydrogen
it
He
+
ions.
in
cooled
is
until
to be
dry
a
tube
very
are
dissolved
phenols
when
-
-
cons
sodium (Nas
as
2)
F0Nat
as
the
in
such
metals
reaction
in
diazonium
with
Reaction
-
left
reactive
2Na
fizzing
some
mixture
-
+
warmed
is
with
vigorously
25500
-
metals:
the
with
lone
density
is
of
phenols:
in
electrophiles
pairs
the
compound (yellow-orange precipitate)
of
electrons
benzene
activating and
compared
ring
directs
on
the
making
incoming
to
benzene
oxygen
it
more
atom
susceptible
electrophiles
overlaps
in-out
to
the
to
with
electrophilic
2.4
and
6
the
is
attack
positions
banding
system
Nitration:
phenols
-
to
mixture
a
give
when
hydrogen
a
-
this
-
atom
known
also
is
2-nitrophenol
of
concentrated
is
the
and
HNOy
benzene
nitration
as
substitution
electrophilic
undergo
can
ring
is
used,
the
product
by
(-NO2)
metro
a
be
will
phenol
of
oh
8
Iote16Nossa
2
dilute
with
nitric
acid (HNOs)
at
room
temperature
4-ntropheno
substituted
is
reacted
when
reactions
is
instead
group
oh
NOz
f
#
S
2-
6-fritrophenal
2, 4,
2
H2 G
Now
nitrophenal
4-Mrophene
S
mixture
products turned
of
ora
oh
ON
PNOcess,
Ore
316, O
↑
2.u.o-trustee
pheno
handl
rominatIOn:
B
-
-
-
undergo
phenols
phenal
this
electrophilic
decolourises
the
also
as
is
known
substitution
bromine
orange
bromination
of
when
reactions
solution
to
reacted
form
a
white
bromine
water
precipitate
of
at
room
2,4,6
temperature
tribromophend
phenol
oh
84
+
with
3Brz
Br
Br
&
3HBr
↓
orange
phenol
solution
B
2,
4,
6
-tribromopheno
(white
precipitate)
Oxidation:
-
-
phenols
oxidation
·
xidising
are
more
can
be
oxidised
easily
achieved
by
than
reaction
alcohols
with
silver
oxide
(Ag20)
or
agents
:Old
·16.
I
C1
Po
Ag. s
01
"
I
NacCrzO7
12
C1
>
4-methylbenzene-1,2
Old
:
>
8 It
:.
bemene-1,4-00/
SOn.
12 0
7
:
:
chromic
acid (NacCre0),
or
other
#
although
this
·
this
*
due
is
compounds
phenol
delocalisation
to
the
increases
· In
phenoxide
the
this
this
·
possible
is
· because
·the
this
·
as
this
of
Htcons
of
phenol
is
the
lone
there
strongly
likely
more
this
·
attached
to
the
·
compared
e.g.
the
·
spread
is
form
phenol
a
is
over
H
out
the
out
the
con
entire
con
delocalised
system
it
the
of
ring
atom
oxygen
as
entire
the
over
with
overlaps
phenoxide
act
the
over
and
on
acid
an
are
rather
less
likely
than
to
to
the
reform
gain
a
phenol
molecule
proton and
act
as
a
basel
("T
caas
become
to
electrons
less
available
bonding
for
with
an
incoming
protein
BASE
stable
more
to
likely
more
position, therefore,
lies
phenoxide
negative
the
to
further
with
con
its
negative
charge
spread
out
conisation
undergo
and
right
and
acid
rather
than
more
stable
a
higher
proportion
of
phenol
molecules
donate
a
proton
ethanol
compound
therefore
is
likely
more
to
act
as
+
cags
phonoxide
phenol
the
the
causing the
low,
cags
right-hand
on
sicreoreside terreia
the
H
· since
ring
to
water
phenol
to
that
equilibrium
aromatic
behaviour
spread
atom
proton (and
a
cags
CONJUGDTE
THE
means
the
into
O
negative charge
courses
acidic
spread
is
oxygen
density
+
phenol
oxygen
charge
lose
to
cags
OF
the
is
oxygen
the
on
less
is
H
3)TABILITY
atom
oxygen
acidia
weakly
are
con
the
on
pairs
&
the
the
from
increases
the
on
pair
not
therefore
and
ring
charge
lone
delocalisation,
are
that
means
one
as
the
of
pairs
phenoxide
the
is
negative
the
one
the
of
lone
they
group,
DENSITY
phenol
of
son,
possible
is
density
CHARGE
base
conjugate
the
the
Phenols
of
COA)
alcohol
an
of
one
of
electron
OF
ELOCALISATION
·
contain
cidity
phenoxide
side
and
on
phenol
(more
formed
is
from
more
the
likely
H
a
base
caas
con
stable)
conisation
to
an
act
as
of
an
phenol
acid
is
rather
than
a
than
base
phenol
itself, the
equilibrium
position
lies
further
to
the
Relative Acidities
pla:
·
the
measure
values
·
order
ELOCALISATION
·
·
as
a
·the
ethand
phenol
phenol
is
stronger and
a
the
looking
their
at
result, the
ethyl
electrons
base
the
is
the
of
the
the
on
the
in
group
result,
a
(which
on
con
ethand
is
base
conjugate
atom
oxygen
the
is
on
the
of
less
are
oxygen
phenol)
available
ethanoxide
electron-donating
an
density
electron
CHyCIc
&
on
the
since
water.
which
are
formed
the
from
disassociation
of
DENSITY
the
016
atom
is
donates
that
more
A *con
a
oxygen
atom
is
spread
out
over
the
entire
density
available
alkyl
-
for
to
band
the
oxygen
formation
stom
with
an
A+
con.
donating
group
(HSC/ 5 0 -
0 caps
H-cass
+
ethanoxide, on
alkyl
base
in
group
to
water
water
of
charge density
compared
con
proton (15 +con)
the
ethoxide
can
hydroxide
con
concentrates
charge
density
on
the
oxygen
atom
which
can
more
easily band
con.
conjugate
the
with
formation
electron
readily
- CHCH2
-
the
on
con
group
th and
electron-donating
167
cags
density
charge
band
for
~
the
and
ethand
bases
conjugate
=) ectron
·
than
table
explained by
be
can
that
shows
and
water
CHARGE
OF
conjugate
·
· as
acidity
phenoxide
the
In
ethand,
of
of
and
phenol,
substance
a
of
Phenol B Ethanol
compounds
he
*
acidity
the
water,
of
acidity
Relative
the
of
Water,
of
the
is,
of
the
the
is
oxygen
phenoxide
therefore,
atom
cannot
become
delocalised
con
a
stronger base
compared
to
phenol
over
a
ring,
the
hydroxide,
on
more
readily
accepts
an
·
as
however,
readily
there
accepts
electron-donating alkyl
no
are
compared
icon
an
water
therefore
is
but
weaker
therefore
further
forther
on
locks
base
than
ethand
position
of
the
·
-
weaker
↓ ydroxide
hydroxide
a
a
ethoxide
the
-A
HO
Water
the
to
the
to
to
an
ethanol
side
side
change
negative
is
Water
concentrated
compared
to
case
the
oxygen
atom
which
therefore
less
ethanol
H caas
+
ring
electron-donating
alkyl
groups
so
water
is
a
stronger
base
than
phenol
lies:
favoring
favoring
cags
and
the
the
disassociated
undisassociated
phenoxide
ethoxide
con
and
hydroxide
cons
CH2CH20'cass +Hicaas
& H cags
hydroxide,
lon
on
I
"
>
-"I
1coas
+
O-
Old
on
con
thoxide
H2Ocis
If
cags
equilibrium
right-hand
CH3CH2-OH
base
less
con
aromatic
left-hand
the
groups,
Ca9)
one
Ali
Nitration B Bromination
compared
·
this
·
because
is
benzene
as
benzene, phenol
to
result,
the
of
readily
more
the
lone
electrons
of
pairs
electrophiles
with
on
the
atom
oxygen
in
overlaps
phenol
with
the
it
bonding
system
the
of
ring
a
there
now
is
donating
electron-
·
one
reacts
Phenol
of
-
increased
an
Of
phenol,
in
group
density
electron
therefore,
in
activates
the
the
ring
benzene
ring
and
directs
incoming
electrophiles
to
the
2.4
and
6
positions
increased
the
·
compared
phenols
↓
reactivity
of
phenol
that
means
different
and
reagents
conditions
are
used
electrophilic
for
substitution
reactions
of
benzene
to
ITRATION
·
It
is
the
an
nitration
~
requires:
concentrated
of
reaction
and
nitric
a cons nitric acid useit
CINO2)
and
sulfuric
acid (H.SOn)
reflux
nitration
290-60°C
at
- benzene
·
benzene
of
mixture
a
substitution
electrophilic
of
e.g.
phenol
of
concnitre
and
at
requires:
temp
room
used
->
occur
only
at
nitration
of
of
phenol,
it
as
is
more
reactive,
so
nitration
conditions
milder
Of
)
NO,
O2N
3HNOs
↳
3H20
diluted
will
>
phenol
NOz
2.
4,5-trintrophenal
Cpicric acid)
the
nitration
·
- dilute
-
·
the
low
phenol
acid
niture
requires:
->
if
form
mixture
a
of
of
on
ortho
basis
the
and
metrophenols
para
their
of
is
further
separated
into
ortho
volatility
Ou
OH
I
ortho
and
para
nitrophenols
temp (298K / 24.890c)
mixture
& stillation
or
9
201.
1NCs
>I
-H20
phenol
-
Lin
"
No,
Atrophenol
intending
are volatieor
tram
&
NOc
4
nitrophenol
fisher sings to")
intermolecular
hydrogen bonding
H20
and
para
trophenols
by
steam
can
ROMINATION
·
substitution
electrophilic
eg.
of
bromination
benzene
of
w/
reacted
- In
the
-room
reaction
requires:
bromine
pure
presence
of
(not
anhydrous
solution)
a
aluminium
[AlBry) catalyst
bromide
temperature
bromination
phenol
of
bromine
water
requires:
->
when
bromine
water
added
is
to
solution
of
phenol
in
water, bromine
water
decolories
observation:
- bromine
-
-
white
product
water
decolories
formed
ppt
smells
of
->
ppt
antiseptic
of
2, 4,
6
trbromophenal
multiplesubstitution acrossthe
on
the
0 H
Br
Br
↓
3BV,
↓
>
3HBr
Br
2.
,6
-
tbromophenol
#*****
come
nitue
and
sulfuric
and
reflux
temp
conc
room
between
250
-
600
nitric
temp
ac
phenols
consist
·
the
·
·
increased
due
the
·
electrophiles
of
bromination
acts
substitution
the
directed
are
by
the
ring
system
is
benzene
the
of
and
ring
become
delocalised
caused
an
ring
benneme
the
the
into
the
ring
hydroxyl
now
is
group
likely
to
phenol
to
more
the
of
undergo electrophilic
the
2,
4, and
6
substitution
and
becomes
activated
positions
phenol:
of
bromine
the
aromatic
ring
density
with
overlaps
PHENOL
ON
benzene
a
electron
atom
density,
electron
to
donates
oxygen
the
in
attatched
group
atom
the
of
density
increased
incoming
e.g.
pairs
electron
the
to
·
lone
(OH)
hydroxyl
this
GROUP
of
hydroxyl
a
in
the
of
one
of
atom
oxygen
HYDROXIL
Effects
* RECTING
of
and
electrophile
as
an
the
hydrogen
atom
can
substitutes
occur
on
hydrogen
a
the
2.4
or
atom
6
the
benzene
ring
position
5
"It
-
ON"
Olf
in
Olf
l
-
i)
>
<
>
<
)
T
&
electron-withdrawing
romination
⑥
the
in
group
and
2, 4
6
directing
positions
Old
O
3
-
REACTIONS
phenolic
of
phenolic
PHENOLIC
OTHER
compounds
·
e.g.
OF
those
are
compound
>
<
&
1
Br
>
contain
phenol functional
a
↓
3ABr
activates
the
Br
COMPOUNDS
that
->
Br,
3
group
-napthol
o
A-napthol
·
· just
like
contains
with
phenol
a
phenol,
group
the-oll
attached
group
to
I
in
benzene
another
mapthal
also
is
ring
electron-donating
and
reactions
the
·
electrophiles
· substitution
I-napthol
·
at
are
directed
the
6
↑ his
carbon
and
other
is
is
to
not
bonded
the
2
possible
to
a
phenolic compounds
and/or
as
there
carbon
react
positions
4
is
atom
in
a
hydrogen
no
of
the
similar
2nd
way
as
atom
on
benzene
phenol
this
ring
carbon
benzene
ring
to
electrophilic
substitution
Production
acids
· benzok
the
-
->
adds
· benzone
the
compounds
& XIDATION
the
·
side-chain
benzoic
heated
is
color
brown
ppt
is
under
with
reflux
Mustcon
-
ofMrOz
acidified
then
such
alkylbenzenes,
in
purple
mixture
oxidation
the
from
reagents
as
is
with
methylbenzenes,
as
solution
a
disappears
they
as
be
can
hot
of
oxidised
reduced
to
to
the
is
oxidising
agent)
Mutcons
formed
and
dilute
(i.e.
HD
sad]
to
Step
the
protonate
organic
product
and
form
produce
side
chains
are
2:Protonation
⑪
0K
t
H
alloteat
-
methylbenzene
>
potassium
benzoic
benzocte
activated
towards
oxidation
at
the
benzylic
position,
because
free
radicals
a re
acid
stabilised
at
that
position
resonance
3 enzylic
Position
->
is
the
next
position
to
a
benzene
ring
the
with
there
should
means
be
at least
one
tert-butylbenzene
C
C
Hs 2
this
carboxylic acid
⑪
alkaline
KMA On
·
a
KMnOn (this
alkaline
are
1:Oxidation
not
through
esters
of
alkylbenzenes
of
CHy
alkyl
synthesis
acid
Step
·
the
in
ALKYLBENIGNGS
OF
alkylbenzene
the
used
often
are
produced
be
accos
carboxylic
C,HgCOOH
derivatives
their
can
alkyl
the
·
·
and
MF:
aromatic
simplest
most
Acid
Benzoic
of
does
hydrogen
not
heat
at
the
benzylic
react
>no
a re
marked
A
-
kMAO4
benzylic positions
asterks)
reaction
position
for
alkylbenzene
to
be
oxidised
to
benzole
acros
a
Reactions
·
acyl
chlorides
Carboxylic
of
functional
-
->
group:
similar
look
-
produce Acyl
to
Chlorides
Coc
structure
in
Acids
to
carboxylic
acids
but
have
cl
a
instead
atom
of
an
of
attatched
group
to
O
carbonyl (c=0)
->
materials
starting
->
they
can
solid
e.g.
from
carboxylic
(v)
chlande
phospharous
(111)
chloride
sulfur
acyl
chlonde
listed
OH
dicklonde
of
CH3
(PCI)
oxide
and
ethanoic
esters)
heat
(ethanoy/chlande)
can
be
formed
and
ethanac
from
C
PCIs
+
>
CH3
ethancy/
C-
in
the
reactions
POCIs
&
Cl
C
5
PCIs
acid
HEAt>
3CH3
ethanayl
cos
acid
+
SOCI
+
HCI
ablonde
O
<
-
x
+
HsPO3
chloride
O
CH3
-CI
(SOCK)
Off
ethanoic
"c
with:
acids
G
3
-
as
(i.e.
compounds
organic
R
used
therefore
(PCIs)
acid
ethandic
so
above
↳O
C
CH3
production
phosphorous
I'guid
acids
the
in
prepared
be
1,quid
->
carboxylic
than
reactive
more
~CH3 -c
ethanoyl
fe
chlorde
&
SO2
+
HCl
OXIDATION
FURTHER
acids
carboxylic
·
the
·
·
alcohols
primary
some
carboxylic
formed
be
can
acids
get
can
alcohols
primary
of
and
aldehydes
to
further
then
to
oxidised
to
carboxylic
acids
oxidised
further
even
oxidation
the
from
oxidised
firstly
are
ACIDS
CARBOXYLC
OF
and
MethanonIce
·
methanol
and
further oxidised
gets
->
reducing agent
strong
->
dioxide (CO2)
carbon
to
O
-on
#
·
the
oxidation
of
warming
and
methanol
-
-
oxidising
purple
orange
oxidising
solution
reagent
solution
Cult
CO2
acidified
turns
kMndy
turns
green
as
as
to
to
cut
you
which
precipitates
as
red
Ag
sadified K, Cr20
or
Mr4
the
reagent
reduced
is
reduced
is
colourless
tollen's
or
on
Age
the
I.e.
Telling's
I.e.
the
->
solution
KCr20,
agents
-
agents
kMnOy
+
by:
occur
mild
with
tollen's
stronger
-
can
febling's
-
using
and
methanol
H20
~
107
+
lons
are
Crotons
are
reduced
reduced
to
to
Mn4cons
crst
cons
thedog
·
·
another
a
strong
carboxylic and
oxidising agent
that
I.e.
can
Warm
get further oxidised
addified
know
ethanedoic
is
dilute
with
is
required
strong
·c
co
+
2
e.g.
(07
add
add
sulfuric
for
oxidation
the
of
ethanedaic acid
oxidising agent
KMnOn/Ht
~
2CO2
2H,0
↑
to
carbon
dioxide
CueO
Method
-
-
It
used
to
oxidised
is
reaction
the
-
a
-
-
-
the
from
can
you
the
ethanediol
of
is
the
manganate (UI)
do
half-equation
half-equation
a
solution
calculation
ethanedion
for
decolonised
is
find
to
and
the
for
the
If
you
combine
can
you
(VII)
solution. You
with
all
with
can't
make
sulfure
up
a
and
solution
actual
until
those
it
5
you
get
concentration
of
to
the
the
end
warmed
point
when
~
con
+81+
2C02
+
and
a
titrated
trace
potassium manganate (vii)
2H+
+
Set
>
of
pale pink
remains
solution
2e-
Mat
+
4H, 8
10C02
+
gives:
(coolt),
+
2MnOi
+
6Ht
·
potassium
potassium manganate (vi)
with
is:
+
of
acid
ethanedoic
sulfuric add,
dilute
with
acidified
solution
is:
manganate (UI)
Mn0i
-
but
acdified
(C80H)2
-
manganate
potassium
concentration,
add
manganate (VII)
burette
a
then
accurated
acd:
potassium
warm
standardise
to
exactly
solution
potassium
by
CO2
used
with
standard
solution
to
is
manganate (vi)
ethanedoic (oxalic)
prepare
2Mn4
+
8H,0
Relative Acidities
·
·
carboxylic
they
acids
act
can
compounds
-
and
acids
as
with
lose
Carboxylic Acids,
of
functional
-cool
a
protonation)
a
B Alcohols
Phenols
group
in
an
ageous
to
solution
carboxylic
form
salts
and
water
aco
->
base
->
O
R
Ot
·
however,
the
·
values
pla
the
the
acidity
Relative
·
this
order
of
STRENGTH
of
·
·
·
·
carboxylic
In
the
electrons
overall,
the
drawing
it
carboxylic
a
conjugate
THe
0-H
in
2-0
towards
acids
Carboxylic
strength
of
a
Water
salt
lies
carboxylic
that
species
of
well
over
adds
an
a re
to
the
left-hand
ands
stronger
side
than
alcohols
table
explained by
looking
the
at
strength
of
the
electrons
its
Ht
are
band
is
in
a re
the
0-A
drawn
WEAKENED
band
due
to
drawn
are
towards
therefore
carbonyl
more
easily
the
100
easier
drawn
o
4
act
band
as
towards
to
an
Acids
2-0
and
band
group
density
from
homeentire
unabals
which
electron
removing
lost
a
compared
proton
to
Phenol
Ethanol
R
CH2-0-H
co
become
acid
bond
band
Carbonyl(2-0)
the
the
towards
group
C
o-H
the
0-l
acids
H
causing
phenals
add
H
R
and
ac
the
adds
carboxylic
the
equilibrium
of
suggest
stronger
the
be
can
of
relative
carboxylate
it
and
itself
can
electron-withdrawing
and
electrons
band
or
phenal
bases
8-Nat
BOND
the
the
value,
position
alcohols
the
of
pla
addities
the
acids,
measure
the
ethanol,
of
relative
of
stability
is
smaller
the
as
acids, phenols and
carboxylic
of
acids
weak
only
are
H20
+
C
sodium
hydrox, de
acids
carboxylic
pla
sodium
acro
carboxylic
O
R
NaOId
#
C
weaker:
no
and
lose
its
c0
group
harder
to
so
act
off
as
an
band
acid
is
and
not
weakened:
lose
t
lackt h ee
the
STABILITY
conjugate
·the
·
·
this
a
as
and
the
·
result,
the
molecule
position
the
of
acids
carboxylic
a
atom
oxygen
charge
electrons
with
IONS
the
on
the
because
is
of
density
charge
the
·
CARBOXYLATE
of
(coo-1
base
the
on
-cool
spread
is
DELOCALISED
is
on
equilibrium
lies
·
the
·
·
as
alkyl
a
alkoxide
·
the
·this
the
conjugate
means
position
the
son
electron
also
lows
bases
that
of
a
to
the
carboxylate
cid
of
-
is
alolide
atom
with
formation
Afton
an
to
reform
undisassociated
compared
right
to
and
alcohols
phenols
"
102
electron-donating
on
the
the
ability
alcohols
alcohols
con
>
an
density
LAck
the
the
bond
for
R-CEO aheeeeeeee
alcohols
the
oxygen
IONS
of
in
group
result,
·
·
base
conjugate
carbonyl
on
-
OA
ALOXIDE
available
less
more
C
carboxylic
OF
electronegative
are
O
STABILITY
carboxylate,
the
over
low
group
disassociation
R
CARBOXYLATE
out
an
atom
oxygen
the
is
are
are
disassociation
atom
oxygen
to
delocate
therefore
Less
WEAKER
equilibrium
the
STABLE
ACIDS
lies
more
is
much
donates
that
group
readily
charge
than
alcohols
compared
to
OHcags
a
Icabal
on
the
the
to
the
band
for
entire
themselves
carboxylic
to
the
R
density
available
density
the
more
electron
and
acids
a
(koxide
(on
formation
with
are
more
and
likely
to
phenols
left
negative
an
Aton
con
charge
ontheoxygen state
R
oxygen atom
is
concentrated
Acas
reform
the
alcohol
STABILITY
·
en
phenoxide
the
In
tire
·
the
a
result,
the
to
the
is
carboxylic
the
phenols
of
therefore
the
of
electrons
on
delocalisation
sons,
position
of
electrons
base
the
since
carboxylate
·
(which
(on
delocalisation
conjugate
however,
IONS
the
conjugate
base
of
phenal)
the
charge
density
on
the
oxygen
atom
spread
is
out
over
the
(on
this
·as
PHENOXIDE
OF
oxygen
is
of
STABILISES
charge
density
phenoxide
disassociation
atom
therefore
ions
equilibrium
phenoxide
the
MORL
are
lies
AVAILABLE
LESS
are
is
(on
STABLE
carbon
on
less
more
than
to
acid,
and
not
on
carboxylate
than
right
the
bond
with
formation
proton
a
compared
electronegative
oxygen
to
alcohols
and
move
The resative charsand breadover
cag)
Phenol
I
&
phanoxide
I
+
Cag
atoms
like
in
the
cons
-
Old
(H+ion)
phenal
atoms
STABLE
for
Hcags
to
the
left
compared
Relative Acidities
electron-withdrawing
·
banded
groups
to
the
of
carbon
Chlorine-substituted Carboxylic
attached
to
the
cool
the
makes
group
Acids
carboxylic
adds
STRONGER
ACIDS
this
·
because
is
weakened
the
as
electron
of
the
carboxylate
Pla
·
values
pla
the
groups,
values
there
are
and
the
on
"
C
stabilised
more
and
even
the
delocalisation
a re
and
examples
less
is
of
likely
acids
carboxylic
ci-ca_
i
Cl
weakest
8-16
bond
0-H
even
further
the
away from
the
on
bond
-coo-group
with
with
an
electron
-
it
on
withdrawing
groups
table
derivatives
and chloro-substituted
bond
dentatives
the
show
stronger
that
the
more
electron-withdrawing
the clean
·T
m PE
strongest
0-H
He-c-10
-
is
negative charge
of the
to
bond
10-16
9--c
molecule
density
electron
more
bonattatotothegop,
the
Cl
extend
and chlorine-substituted
ethanoi
of
O
"C
draws
group
groups
acids
carboxylic
ethanoic
of
withdrawing
even
now
is
chlonne-substituted
·
and molecule and
undisassociated
the
on
the-coo-group
·
-
in
electron-withdrawing
furthermore, the
·
o-band
the
more
the
acd
chance
atoms
carbongleads,
is
there
the
are
stander
thePhilli
in
monset
p
and
·trichloroethanoi
the
amend
when
the
o-H
withdrawing
is
the
from
broken
to
is
and
as:
the
WEAKEST
-cool
the
form
since
there
are
three
strong
very
electronegative
(I
atoms
IS
10 U
carboxylate
STABILISED
so
that
(-C08-1
the
con,
density
charge
is
further
spread
out
by
three
electron
is
less
attracted
to
44
cons
Cl
Cl
~O
-
che
CI
·
acid
ethanoic
is
the
contains
A
the
methyl
Cl
Lot'sit
⑧
weakest
an
group
Is
acid
strong
0-
H+
-
Of
chargedensity isspreein
bad
as:
electron-donating
negative
methyl
group
which
STRENGTHENs
the
charge towardsthecoo-group which
O
-coethanoic
add
DONATES
·_10
d
a
bond
withdrawing
group
atoms
21
carboxylate
the
strangest
CCIsCOOK
in
density
electron
the
is
is
ethanoate
↳
(on
Increased electree
0-H
band
becomes
more
likely
to
accept
on
to e
-
Production
·
Esters
->
->
->
->
->
->
they have
used
can
in
be
alcohols
more
characteristic
prepared
effective
and
e.g.
of
way
the
acids
condensation
reactions
esterification
go
alcohols
and
O-0-R
carboxylic acids, acyl
chlorides
alcohols
completion
(so
no
+
adds
carboxylic
reaction)
chlorides
equilibrium
mixture
->
are
is
esters
reactive (so
more
formed
(condensation
and
the
yield
reaction)
the
of
reactions
the
ester
happen faster)
is
include:
reactions
chloride
ethanoyl
to
between
acyl
esters:
with
reaction
(esterification
esters
->
reactions
their
group
chlorides
acyl
solvents
as
preparing
of
with
R.
and
the
from
carboxylic
+
functional
COR
an
smells
cosmetics
perfumes,
unlike
->
with
compounds
organic
by reacting alcohols
of esters
ethano
>
ethyl
ethanoate
16
c-c
i
16
ethyl
H-o-"a-c-x-p-b-d-o--
#
↓
eth and
ethanoate
benzoyl
chloride
+
phene
is
is
>
phenyl
o
Il
S
benzoyl chlonde
phenol
a
) coh ol
HCl
I
benzoate
-<#
chloride
16
ethanoate
↓
C-c
↓
ester
16 D
o
/
acyl
ethyl
aloot of
Acyl chloride
-
&
O
phenyl
esters
benzoate
O
&
HCI
maximum)
Reactions
·
chlorides
Acyl
reactive
->
·
addition
In
by
·
-
elimination
of
reactions
many
addition
addition
le
reactions, the addition
small
a
these
of
eg.
elimination
compands
organic
undergoes
->
Chlorides
Acyl
of
of
small
a
elimination
-
molecule
reactions
the
across
bond
20
place followed
takes
molecule
-
elimination
include:
reactions
hydrolysis
chlondes
acyl
chlondes
acyl
alcohols/phenols
+
>esters
ammonia/amines
+
amides
>
HYDROLYSIS
hydrolysis
the
·
·this
addition
an
is
-
hydrochloric
a
e.g-hydrolysis
results
elimination
molecule
water
a
·
chlorides
acyl
of
of
formation
in
of
a
and
carboxylic acid
HCI
molecule
reaction
adds
the
across
acid (HCI)
molecule
propanoyl
chlonde
C10
bond
ELIMINATED
is
to
and
propanoic
form
+
HC
carboxylic acid
->
CHe-CH2-ca
propanay)
He O
Chy-CH2-CO
·
&
P
↳
C
acid
propanaic
chlande
O
1428
t
1)
&
Hl
&
1641
C
I
16
3
CH2C
soo
-
16
3
CH2C
Hy CH2C
CI
O
S
16
Go
C
3CH2C
t
3
Nucleophilic
1
stage:
by
↓
a
nucleophilic
of
one
attack
lone
the
Addition
on
pairs
on
Reaction
fairly
the
the
positive
oxygen
carbon
of
a
atom
molecule
water
o
O
16
CI
involves
I
I
Old
do
CH2C
t
I
1y CH2
C
(
~
by CH2
C
-x
4)
240 stage:
i
1)
Elimination (2 steps
carbon-oxygen
double
band
reforms
and
a
chloride on
is
pushed
off
↓
18
-x
is
C1+
~
Hy [16,
C
Lo-16
2)
this
give
H 2
is
followed
ethanoic
by
and
removal
and
of
hydrogen
hydrochlone
ion
ac
by
the
chlonde
on
to
FORMATION
ESTERS
OF
chlondes
·acyl
·
esters
formed
phenal
+
less
are
HCI
+
120
+
ester
->
reaction
the
from
acids
carboxylic
as
chlorides
acyl
therefore
are
esterification
the
·
chloride
be
ester
alcohol,
+
carboxylic
of
and
reactive
acids
alcohol
the
chlorides
addition
an
the
across
-
base)
alcohols
CH2
e
c
elimination
·
Ot
Nocleophile:
-
-
both
the
they
both
It
is
oxygen
pull
that
and
chlonne
electrons
is
carbon
atom
which
the
one
attacked
either
are
molecule)
or
leaving
negative
which
amount
has
active
atom
two
the
carbon
atom
the
carbon
atom
one
of
the
lone
negative charge
of
lone
pairs
on
the
CH2
and
so
the
a
somewhere
drags banding
oxygen
of
region
on
a
electrons
to
attack
molecule
either
is
from
also
above
planar (flat) around
or
below
the
plane
that
of
carbon
the
-
CI
y
positive charge
in
molecule
toward
itself.
atom
electrons
of
the
in
quite
pairs
on
COCl
group
positively
the
are
very
charged
oxygen
atom
is
8-
electronegative
an
ethanal
City-C12-C
T
easy
chlande
propancy)
nucleophile
to
8-charge
strongly
carbon
Ha'cg(
+
chloride
ethanoyl
the
a
and
hydrogen
than
molecule
-
c
"
propanote
attracted
strongly
is
have
else
or
ions,
significant
attacks
to
fully
electronegative
more
a
these
by
species (ion
else
a
much
oxygen
of
themselves,
is
CH2
ethyl
something
produces
this
attatched
atom
towards
is
oxygen
-
-
-
Chy
~
-
~steamycoilsare
esters
-
#
-
Ot
-
eth anal
-
product
less
bond
c-0
CH2
Cty
:.:-nocleophiles
Cha Cha
slower
ELIMINATED
is
#
-
a
is
reaction
chlande
propanay)
this
esters
of
->
CH3
however,
metmementon (so
synthesis
the
in
also
is
adds
phenol
or
molecule
HC)
a
acyl
of
useful
more
phenols and
with
reaction
the
and
heat
(requires
formed
is
·
also
can
reaction
chloride
acyl
-
with:
react
can
Acyl
-
atom,
molecule
and
that
leaves
plenty
of
room
for
by
a
attack
S
nucleophile
C1
0-
NUCLEOPHILI
ADDITION
STAGE
the
as
to
lone
form
ethanal
an
-
the
on
pair
band
a
that
onto
C
with
it.
the
In
leaving
oxygen,
approaches
oxygen
the
process,
the
electrons
two
in
carbon
positive
fairly
one
3
C.
the
in
ethancyl
bands
are
chlande
propanoy)
chloride,
it
moves
repelled entirely
·
↑o
CH-CH -
the
in
carbon-oxygen
the
of
carbon
the
to
charged
negatively
it
attatched
becomes
molecule
CHCK-C
CI
co
+
<Hy 2H2
"g
CH3 [He
16
H
the
the
lone
Why
-
charges
the
looks
-oxygen
car ries
the
the
You
up
from
underlying
the
reason
for
this
what
is
when
oxygen.
oxygen?
start
with
moving away
are
The
with
a
overall
two
negative
on.
There
and,
molecules
neutral
has
to
got
be
a
if
the
forgot
you
positive charge
positive
somewhere
to
equation.
wrong! Oxygen
normally
forms
two
bands, but
here
its
forming
three.
Oxygen
back
to
only
can
form
three
bonds
the
carbon,
charge
steps
STA9f-2
of
in
positive charge.
a
carbon, electrons
the
used
end
would
band
double
carbon-oxygen
pair
with
charge
positive
a
ELIMINATION
bond
gained
has
ethanal
don't balance.
you
the
the
in
a
positive
is
balance
1)
forms
pair
charge,
it
atom
oxygen
electrons
the
in
reforms.
As
the
electron
band
carbon-chlorine
moves
pair
forced entirely
are
form
onto
a
band
the
with
chlorine
to
give
a
·
chloride
con
:CI
S
CI
CHCK-C
>
C
Chy CH2
-CH2CH3
+
16
<Hy 2H2
16
2)
finally,
the
electrons
CHy CH2
in
chloride
the
on
forms
a
band
hydrogen-oxygen band
C
->
CH2CH3
T
16
with
more
the
back
&)
hydrogen
onto
the
CHy CH2
on
the
oxygen-removing
oxygen,
C
cancelling
-CH2CH3
<
:CI
#Cl
the
it
as
a
hydrogen
positive charge
low.
The
if
~
Old
CH2
CHo
Chy
NaOld
#
C
CH2
C
+
>
heat
ester
Nach
+
128
Cl
phenal
one
·
phenyl
with
reacts
between
chloride
propanay)
needs
& ENERATING
THE
to
temperature, although
room
together
make
NUCLEOPHILE
the
reaction
isn't
the
hydrogen
with
reaction
chloride
gas
faster
-
o-Nat
&
heat
NaOH
-
H2O
3
↑
sodium
phenal
phenoxide
ADDITION
NUCLEOPHILIC
CHyCHe
at
propanoate
alcohol
formed
is
modified
be
chloride
propancy)
propanoate
phenyl
·phenol
phend
chloude
propanoy)
"C
propanoy)
-
STAGE
Nat
·
-Nat
D
>
chlonde
sodium
Chy
-
CH2-4-CI
phenoxide
lon
ELIMINATION
8-
Che-CH2-"
Nat
-'C
>
Chy-CHe-c
g
phenyl
propanoate
+
NaCI
as
fast
as
the
FORMATION
chlorides
acyl
·
acyl
·
AMIDEs
OF
+
chlorides
1
+
>
ammond
non-substituted
/ 2 "amine
amide
amide
substituted
>
->
CH3
C
CH2
ablonde
propancy)
NUCLCOPHILIC
Ntsa
f
Cl
CH3
s
CH2
-
c
propanamide
"Wiz
amide
+
HCI
ADDITION
go1
of
ChgCH2-C-C)
-
Chy-CH2-E-c
is-N
propancy'
-
chlande
i
NH3
·
involves
the
the
atom
nitrogen
attack
nucleophilic
in
the
the
on
fairly
positive
carbon
by
atom
the
lone
pair
on
ammonia
ELIMINATION
:
1)
'C
CH3CHe
1s-N
↓
CI
>
(HyCHe-c8:
N
-- $
I
band
carbon-oxygen
a
chlonde
ion
ChyCHe -c
followed by
is
>
1-N-$
is
removed
ammonia
to
by
give
the
a
pushed
g
"
its
this
-$
is
O
2)
It
and
reforms
N
ammonium
-
D
is
·
9I-
removal
chloride
off
#CI
C
ClsCH2
CI
on,
chlonde
of
hydrogen
producing
HCl
low
from
(which
the
would
nitrogen
immediately
react
with
excess
amide
->
->
&
C
CH2
+
C
CHs
NH
mIt
Chy-CHe
>
2
methyl
methylamine
chlonoe
propancy)
a
primary
ADDITION
NUCLEOPHILIC
Che -CH2_
-c@
propanamide
+ If C
NA
Cy
G
eOu
Che CHe
~
Phs
c-C)
16
N-A
-
N-CHe
↓
I
ELIMINATION
8:
·
c
CHyCHe
cI
-
is-N--cl
ACID-BASE
"-NH-CHs
Chy CH2
>
methylpropan
+
HC
<mide
REACTION
↳
same
nucleophile
NHcCHy
AD
acid
CH, NHy
~
&
base
ammonium
<
21-
salt
substituted
->
secondary
-
Chy-CHe-cY8
Chy-N16
↓
CI
propancy)
>Cho-cHe
-CHe
amide
-Y-NH-cD3
diy
ADDITION
NUCLEOPDILIC
CHy CH2
amide
o
·Oo
>
Clg C12
-
CI
-NCHs
Cy
chloude
c
⑪Do
N-Chg
·
A
E
LIMINATION.
<He C162
Is
CID-BASE
A
T
-Nice
C
C
& I
>CsCke
G
Ntcl
dimethylpropanamide
REACTION
↳same nucleophile
C
↓
NA (216
base
C
~
C216
>
N16,91-
ammonium
salt
+
Ac
+HC
Hydrolysis
·
·
Hydrolysis:
the
·
e.g.
ease
the
breakdown
of
hydrolysis
for
of
ease
compound
a
hydrolysis, starting
of
this
trend
STRENGTH
·
this
there
the
·
C-D
of
c-cl
bond
therefore
is
to
electronegative
strong
at
and
atom
nucleophilic
is
away
also
from
attack
of
attatched
to
an
atom
oxygen
the
carbonyl
carbon,
the
carbonyl
carbon
leaving
much
is
it
very
more
8t
RAPID
electronegative
two
a
temperature
room
electrons
pulling
atoms
deficient
electron
BOND
CCI
THE
chlorine
the
WEAKENED
very
OF
Is:
chloride
anyl
readily
most
banded
carbon
chlonde
down
broken
CHLORIDES
ACYL
IN
readily
most
STRENGTH
3
HYDROLYSED
the
two
are
BOND
a re
because
is
explained by
be
chlorides
acyl
·
can
alkyl
chlonde
Halogenoarenes
differ
may
compounds
with
Chlorides and
water
using
different compounds
acyl
·
Acyl Chlorides, Alkyl
of
tOMS
C
heat
R-0C,
acyl
STRENGTH
·
the
·
the
·
the
·an
atom
carbonyl
carbon
C-C1
OF
atom
hydrolysis
OH-con
is
~weakened
hydrolyse
7
not
very
the
electron
-
alky)
CI
C
chlande
-
8
a
chloride
H 2I
1
Of
acid
carboxylic
bond
(-0
RC
CHLORIDES
attatched
only
and
requires
alkyl
>
temp
the
C-C
it
is
bond
one
is
ALKAL1 (i.e.
STRONG
as
to
a
STRONGER
electronegative
stronger
OH)
to
atom
than
be
which
the
refloxed
NUCLEOPHILE
with
than
strong
Nacht
heat
>
R
c
OH
+
pulls
Gal
deficient
~relatively
R
ALKYL
chlorides
alkyl
therefore
IN
alkyl chlorides,
of
will
chlande
BOND
in
room
ci
NaCI
He O
it
electrons
bond
in
away
acyl
from
chlorides
it.
STRENGTH
·
in
anyl
OF
BOND
C_C
chlorides,
the
IN
carbon
ARYL
CHLORIDES
bonded
atom
to
the
chlone
C1
atom
atom
is
part
of
delocalised
the
to
bonding
system
ring
·one
·
·
of
the
as
the
CCI
a
lone
bond,
result,
the
pairs
of
electrons
has
therefore
c-cl
band
is
of
some
the
double-bond
difficult
to
break
OVERLAPS
character
and
causing
hydrolysis
>ccIhaspartial poles
strong
very
2)
H 8
2
3
no
hydrolysis
reactIOn
aryl
chloride
delocalised
with the
will
it
to
not
become
occur
system
STRONGER
of
the
benzene
Production
·
·
Primary
amines
Secondary
-
compounds
organic
I
amines
have
two
R
that
alkyl
Calkyl)
have
any
or
NHe
an
groups
containing
amines
attatched
group
composed
the
to
than
basic
more
no
of
one
20
than
considerable
any
nitrogen
alkyl
one
or
group
atom
bonded
atom
and
amines,
ammoning
stent
hindrance
but
R
NH2
amine:
groups
composed
the
more
no
alkyl
nitrogen
basic
the
to
two
of
including
or
-Nin
atom
nitrogen
groups
all
amine
amMonia
stent
hindrance
NH
alky)
an
anyl
or
group
grow at
is
R2Cary
30
bonded
forms
attatched
the
the
to
composed
to
Amines
containing
any amines
atom
that
considerable
R
an
to
R, NH-Re
I
attatched
alky)
two
R
-
to
attatched
Amines
containing
amines
to
go
attached
secondary
20
alkyl
the
to
nitrogen
basic
less
amine: R-NHe
AMINES
group
8-y
If
10
functional
groups
i-n
*
primary
Amines
Of
of
nitrogen
basic
than
but
less
basic
stent
alky)
nitrogen
R
anyl
groups
atom
groups
bonded
atom
ammonia
than
hindrance
R
R
or
alkyl
three
more
shows
three
N
and
20
10
amines
amines
40
amines
prepared
be
can
different
from
including:
reactions
#
halogenoallcanes
-
halogenoallcanes
-
reduction
-
REACTION
·
the
-
lone
excess,
-under
pair
Conditions:
not
ethanoic
AMMONIA
WITH
SN2
-
acts
ammonia
in
B
amines
nitriles
of
reaction
substitution
nitrogen
Reagents
10/20/3%/40
+
PRIMARY HALOGENOALKANES
OF
nucleophilic
·
ammonia
amides
of
reduction
-
+
NUCLEOPHILE
a
as
replaces
under
reflux,
the
halogen
halogenoalkane
in
ammonia
pressure
-carried
out
in
sealed tobe-mixture
a
escape
could
up
the
not
heated
be
condensar
as
I
<-c
s
⑪
Lethanol)
Mechanism
-
nucleophilic
two
would
ammonia
simply
+
NAnC
is
is
ammon (a
the
A
>H-9-c-NHz
hect
2NH3
because
gas
a
16
+
Chloroethane
1)
and
ethylamine
(10 amine)
step
substitution
City
-
SN2
reaction
-
as
"CH2 -co-
-
it
involves
the
>
collision
Hi
- I
the
2)
1
s
H
N=1N-16
i
two
reversible
remove
on
one
the
molecule
of
the
may
hydrogens
step
of
the
reaction
io-is
more
mixture,
the
favoured
reaction
there
ammonia
more
the
is
forward
the
in
reaction
is
Che-Ct
#
16-N,!
is
is
ammonium
slow
+:C1
ethylamine
=>
an
the
in
species
Chy -CH2
is
CHy -CHe
of
$
IO
GMRGIUM
S
product
-N163+
->
mixture
of
ethylammonium
ethylamine
major
product
-
only
and
be
lons,
ammonium
ethylamine
ammonia,
lons
if
ammonia
an
every
as we
OF
both
SN1
and
SM2
content
(not
required
an
·extended
REACTION
SECONDARY
Conditions:
excess,
not
ethanoic
-under
AMMONIA
WITH
SN1
-
B
Reagents
-
HALOGGNOALKANCS
substitution
AMMONIA
WITH
mechanism
TERTIARY
OF
nucleophilic
·
HALOGGNOALKANCS
REACTION
ammonia
pressure
-carried
out
in
sealed tobe-mixture
a
escape
could
up
the
not
heated
be
condensar
under
as
reflux,
NHy
+
CI
CHg
ammon,
the
ammonia
would
simply
gas
a
CHy
CHe-k
because
~
Cls-
Niz
+
NAnC
⑪its
a
2-chloro-2-methylpropane
2-methyl
propylamine
-
130 ame)
MECHANISM
initial
involves
consation
of
halogenoallcane
the
CH3
CH3
cis-c
followed
by
cls
a
s
cls
s
slow
&I
CH3
-4
:21-
&
I
CHy
rapid
very
attack
by
the
ammonia
16
CH3
16
C
No-16
I
N-H
fast
the
on
I
>
CH3
is
Cs
16
i
CH3
H
C
N:
I
N-H
is
carbocation
CHy
CH3
If
16
A-W-$
is
(carbonium
ion)
formed:
REACTION
·
·
·
HALOGGNOALKANCS
OF
nodeophilic
nitrogen
the
in
excess,
-under
primary
B
Conditions:
not
ethandic
Reagents
-
substitution
WITH
PRIMARY
AMINES
reaction
acts
amine
primary
as
a
nucleophile
and
replaces
halogen
the
the
in
halogenoalkane
amine
pressure
carried
A
out
in
sealed
a
to
be
b
I
16
Hc-<-N-1+
I
!
is
ethy)
ChyCIeCI
--"_-x+Aa
I
heat
Lethanol)
i
b
chcroethane
amine
diethylamine
(10 amine)
(20
amine)
MECHANISM
Chlorine
a
lost
is
chloride
as
(on
&·
che
-
CHe
-
8
Cha-Chy-i 3 onePC
16
CH3
-
CH2
N-$
CAs-C12 =
is,
so
~
onthenitrogenre
Chy
citz
-
3
-CHe:D-
I
CH2 -N-$
is
ammonium
chloride
in
to
which
two
the
of
hydrogens affatched thenitrose
cho-chees 3 diethylamideben 2alkyl
are
groupsin
16
-$
chlonde
sethy ammonium
*N=-$
i
REACTION
·
·
HALOGENOALKANE
OF
nodeophilic
conditions
-
excess,
not
ethanolic
-under
SECONDARY
AMING
reaction
substitution
B
Reagents
A
WITH
secondary
amine
pressure
carried
out
in
sealed
a
to
be
I
It
16
↳<-c-w-d--x+
i
⑪
*
I
I
b
i
isit
CHyCHeCI
heat
~
eth anal
i
Chloroethane
is
is
(20 amine)
20
16
is
triethyl amine
amine)
230
dethylamine
MECHA NISM
is
1s-9--6---4+1C)
amine
lone
pair
atom. Then
has
on
it
active
an
nitrogen
the
will
CH3-CH2-18-bromoethane
chlorineisdispense
attack
if it
collides
3
CH3-CH2-N-CHe-city
Chy -CHa
: [I-
Chy-CHe--N-CHe-ct
S
trethylammonium
chlonde
che
alls-the
CH3
-
CH2
-
N-CHa-cIy
is-N
s
Chy
-
-
CH2
I
CHe-N-CH2
-16
x
"N
i
=
triethylamine
C30 amine)
$
-CHe
&.
I
os tIILOGCNOALKANG
A
WITH
TERTIARY
AND
-
-
no
B
conditions
not
ethanolic
Reagents
·
-
excess,
-under
secondary
amine
pressure
carried
out
in
sealed
a
to
be
If
cites--
I
isit
16
is
id---c-c-x+CHyCHeCl
i
is
is
Id
ethanol
is
Id
>Hi
heat
+
-
⑪
1sc
triethyl amine
amine)
230
-
$
is
etraethylammonium chlonde
↓
the
MECHANISM
-
amine
on
pair
and
attacks
has
the
the
active
an
nitrogen
carbon
of
chorethane
the
*CHe -pW-M
CH
CH3
30
lone
closed-c42-c
-
3
CH2-N-CHe-CHe
che-He
CHy -C12
there
is
atom
on
no
the
·nucleophilic
primary
AMIDEs
OF
substitution
acyl
amide
·secondary
amide
·tertiary amide
·
Reagents
-
LiAlly
the
4(H]
+
+
>primary
4C]
the O
amine
>secondary
tentary
- >
f
amine
1628
+
amine
16,0
conditions
B
in
10
dry
ether
A
A
Amnes
C
Alta
↳
N
>
R--N
A
R
10
A
2
Amnes
R
a
↳
N
20
30
All
a
>
R-k-N-R
&I
↓
R
amid
20
amine
e
⑪
Amines
amine
amide
10
R
R
N
↳
3:
amid
e
↳
comes
addition
nucleophilic
4(1]
+
reaction
so
longer
nitrogen
molecule
ammonia
REDUCTION
:CI-
All
a
_N/R
>R
-
3°
amine
R
a
could
to
hydrogen
that
an
an
remove,
end
:CI-
1
AMIDE
10
TO
AMING
reducing
H-d-eNts
eth an
has
a
I
I6
proton
that
attacked
connected
is
by
to
acidic
the
If
~4-k-c-w-
us t
-
is
+
ethylamine
the
enough
hybride
to
i
Hy (
Lit
i
@
A
"Lit
S
H
Ally
Al
·
H
wH
>He C
deprotonation
>
Lewis
↳it
:
AA
Reaction
a.eresaontherefilingele
Lit
Lit
If
"Filte
Acid-Base
WH
Is C
:"AIHy
-A
I
&
HyC
3
Lit
④
>
$20
I
↓
amide (acetamide)
nitrogen
be
agent
CLiAlHy)
"wil
He
c
WA
is
-
<
LizA1Hy0
HyC
NH
I mile
nucleophilic addition
theNewtrial
we
2:
AMIDE
TO
S
2:
Hydeis
Hii-"
If
&
wit
Hy C
acidic
HyC
WHe
mine
workup
AMING
-N--x+Ux
Hi--N-c-x+HeO
A
*
i
is
N-methyl ethanamide
mechanism
>
a
↓
.
same
Sil
·
i
is
1-methylamino ethane
10
as
to30Amr
e
·
e
T
is
N.
Chy
N-dimethylethanamide
16
sCPS
>
If
Hok-d-N-CIs
I
1,
1-dimethyl
amino
ethane
ce
cross
T
band broken
/
As C
↳i
I
Alz
bonds
CNce) bond formedan
broken
darmer
T
(there;
Then
t
is
H
broken
'NIC43
a bond formsan
che
>
Is C
Reagents
-
Hy
in
solution
by
in
4
t
At
takes
Al
with
isn't
it
primary
strong
dilute
acid
>H--"-NA
is
is
carbon-nitrogen
a
a
triple bond
is
reduced
to
ethylamine
give
amine.
enough reducing agent
to
reduce
hydrogen
of
metal
catalysts
(palladium, platinum,
nitriles
mckel)
gas
16
H-*-cN
I
ethanenitrile
+
2H2
Ni
Catalyst
>
Id
H--c-N1z
is
is
ethylamine
available).
tetrahedral
intermediate
intermediate yields
conditions:
B
-presence
-
reaction
(167
a
Reagents
that
↓
the
-
of
reducing agent
CLiAIHnT
-c=N
NaBty
product
the
of
162
tertiary
aldehydes.
elimination
not
kept cold, hydrolysis
ethoxyethane (ether)
treatment
!
ethaentle
-
this
of
is
If
conditions:
B
followed
x
⑪↓g
initial
stable.
loss
N-D
no
NITRILES
Of
LiAlly
-
N:
Cha
H;Al-H
REDUCTION
of
I
I
the
fairly
NC) band
C-
by
accisted
diy
amides,
step
elimination
N-CHy
16
is
133
the
2
43C
1s-Hi- $
Li
tertiary
for
7
C-0
"
nei
*
t
higher
place.
temperature,
Production
Amides:
·
·
·
·
chloride
acyl
+
condensation
in
this
In
reaction,
·
·
the
chlorine
the
carbonyl
carbonyl
·
·
atom
chlorides
ammonia
condensation
and
together
join
on
together
to
and
draws
and
ammonia
electronegative
is
electron
therefore
is
in
the
product
the
is
however,
and
deficient
has
amines
reaction
is
be
is
amide
substituted
a
can
lone
formed
slower
not,
or
and
from
pair
reaction
the
in
form
an
process
eliminate
amide
and
eliminate
density
from
the
HCl
an
electron
CH3
be
attacked
by nucleophiles
of
electrons
which
can
act
as
a
molecule
and
nucleophile
depends
produce
ammonia
condensation
the
the
on
acids
are
a
nature
the
of
the
attack
nodeophile
amide
non-substituted
reaction
less
amide
between
reactive
acids
and
chlondes
and
carboxylic
than
anyl
CH2
-
c
-
chlonde
f
Cl
NId
~
Chy
the
~
-
CH3
CH2
-
chlonde
Propanoy)
-
Cl
t
CH2
-
City-N16c
Methyl
10
chloude
acyl
CH3
c
c
t
acyl
see
20
a
go
to
pg
6
a
Mide
t
If
CI
~
-
awide
substituted
->
Chy-C12-
dimethyl
amde
-N-Cy
d
amin e
mine
substituted
->
-c18
30
chloude
mechanism
20
-C1y
CI
NHz
N16 -C y
methylpropan amide
amine
dimethyl
chlonde
If
chide
Chy-c1,
amine
City-N16
Cl
Propanoy)
>
amide
&
propanomide
go
O
CH2 -C
10
-
dosen't
reaction
-
chloude
acyl
amines
ammonia
->
Propanoy)
to
molecule
carbon
carbonyl
completion
to
small
a
formed
is
substituted
a
carboxylic
as
amide
an
will
amines
chlandes
acyl
and
broken
secondary
of
also
can
this
a
and
reaction
amides
bond
c-C)
primary
·
and
3
amide
molecules
organic
group
carbon
whether
·note:
two
acyl
in
carbon
result,
a
as
functional
CONR2
>
chloride
acyl
atom
nitrogen
the
-
REACTION
CONDENSATION
·
an
amine
ammonia
the
case,
with
compounds
organic
Amides
of
↓
prop
amide
an
3
amide
If
&
CI
BASICity
·
·
the
Actors
of
nitrogen
they
atom
DATIVE
a
Solutions
act
and
BASES
as
Amnes
of
Amits
of
ammonia
in
therefore
can
form
Basicity
molecules
amine
by donating
solutions
ageous
in
accept
can
proton (H+on)
a
lone
its
electrons
of
pair
to
and
proton
a
BOND
undergoes
ammonia
e.g.
an
NIz
ACID-BASE
REACTION
HCI
>
-
a
base
cid
NHy"
with
hydrochloric
all
acid
(HCI)
to
salt
a
form
CI-
salt
AMMONIA
NHy
+
H
-
ND
>
AMINE
PRIMARY
R
NHz
16+
+
NAy
-
AMING
SECONDARY
R
N
R
H+
+
Noct
3
Re
R
STRENGTIS
·the
AMMONIA
OF
strength
form
a
·the
more
of
dative
readily
positive
B
depends
amines
covalent
this
inductive
lone
& elocalisation
-
the
bond
effect-
presence
some
eg.
lone
pair
ethylamine
ring)
into
(which
an
availability
the
is
such
electrons
aromatic
electron-donating
alkyl
become
to
(e.
rings
form
available,
as
benzene
to
of
the
lone
pair
electrons
of
the
on
to
atom
nitrogen
proton
a
groups
available
become
less
has
the
electrons
of
of
BASES
on
with
pair
delocalised
the
AS
AMINES
of
·
R
>
the
groups
rings
THE
BASE
Is
monensity mothngen
of
atom causing
and
available
more
benzene
STRONGER
causes
the
therefore
lone
pair
increasing
of
the
electrons
the
lone
pal in
basicity
amine's
on
the
nitrogen
atom
to
be
ring
a
dative
ethyl
covalent
group)
is
band
more
with
basic
ammonia
than
and
hence
phenylamine
decreases
(which
has
the
an
amine's
basicly
on
electron-withdrawing
benzene
Preparation
Phenylamine
·
It
produced
be
can
compound
organic
-
Step
1-
three-step
a
in
consisting
Nitration
of
synthesis
benzene
-
reaction
to
an
separation
by
nitration
form
and
ring
followed
undergoes
25-3000
at
benzene
a
Phenylamine
of
with
come
amine
(NAz)
functional
phenylamine
of
nitric
reaction
from
and (HNO3)
group
and
mixture
sulfuric
cone
and (HysOn)
untrobenzene
NOe
He O
3
HNOz
+
4
nitrobenzene
benzene
(reduction)
Step
2
-
Reduction
-
ntrobenzene
to
form
reduced
is
acidic
an
with
mixture
tin(s)
hot
that
contains
and
the
come
organic
NOz
T
(B]
3
-
+
Deprotonation
-
4
-
(Nadlt)
is
added
&
↳
to
NH3
the
He O
con
acidic
reaction
mixture
to
form
NHe
Olt-
t
Step
hydroxide
reflux
NH3
Phenylamine
sodium
under
CgHsN'Ha
3
netrobenzene
step
product
#
+
and (HCI)
hydrochloric
Separation
-
&
the
phenylamine
21628
4
>
is
separated
from
the
reaction
mixture
by
steam
-
NHe
vipinoNiiMm
⑧he
-
oomano
>
distillation
phenylamine
Reactions
both
·
ACTIVATION
·
OF
the-N1c
than
eg.
it
the
dazonium
a
-NHe group
phenylamine
in
can
attatched
will
the
to
benzene
ring
phenylamine
in
·
a
react
will
rings
only
the
on
with
an
solution
ageous
with
bromine
touches
the
react
nitrogen
or
·
in
the
other
delocalised
becomes
benzene
effect
chemical
reactions
has
of
making
the
ring
much
reactive
more
of
bromine
(bromine
water)
in
the
cold
and
in
the
absence
catalyst
pair
and
the
in
be
in
the
presence
delocalised
electron
..
part
call
undergoes
slightly
words,
substitution
positive parts
it
undergoes
of
with
a
catalyst
electrons
...
ung
↳
them:
reactions
in
which
molecules.
electrophilic
of
and
overlapbetweenthe
·
take
RING
otherwise
any
lone
the
as
phenylamine
THE
phenylamine
inactivated
·
of
group
would
of
·
of
formation
-
well
as
amine
include:
bromination
-
·
ring
reactions
these
·
benzene
the
Phenyl
of
substitution
the
ring
electrons
are
attacked
by positive
cons
·
If
you
DIRECTING
THE
·
·
·
·
the-NHe
that
the
you
·
the
will
·
·
·
-
is
OF
phenylamines
react
the
lone
the
pair
of
will
-NHe
incoming
electrons
extra
involving
the
from
-Nte
group,
I
becomes
GROUP
activating
the
by
ring
effect
into
go
tend
will
groups
2,4-directing
a
to
than
ring
faster
others
they
than
will
others
into
effect
2-position (next
the
into
go
the
much
positions
some
has
group
around
positions
some
on
door
to
the
-NHz
group)
or
the
4-position
group)
the
of
any
3-isomer
formed
-
it
is
produced
slowly
too
PHENYLAMINE
electrophilic
in
electrons
phenols,
the
on
oxygen
the
delocalisation
of
the
benzene
therefore,
the
incoming
ring,
NHz
groups
that
NHe
BROMINATION
-
more
hardly
get
THE
the
electrophiles
incoming
incoming
that
means
in
·
has
effect
net
that
·
the
OF
around
density
electron
to
EffECT
group
means
Copposite
·
attractive
more
even
the
increase
the
electrophiles
phenylamines, therefore,
↓is
the
atom
donates
atom
caused
becomes
activated
directed
to
under
reactions
nitrogen
electrons
are
react
substitution
milder
the
lone
increased
an
and
conditions
becomes
and 6
with
similar
a
pair
electron
more
datelemtymhm
t
bkening
density
instead
in
benzene
the
attacked
readily
by
ring
electrophiles
positions
ageous
bromine
at
room
temperature
to
form
2, 4, 6
NH2
Br
3Bracag)
PHENOLS
as
electrons
of
advisinga
&
way
phenylamines
in
its
2.4
in
&
phenylamine
2,
B
r
I
u,6-thermophenylamine
+
3HBr
-
tubromophenylamine
FORMATION
Dazonium
·
the
to
of
compounds
dilute
·
these
dazonium
group
withe (111)
and
(i.e.
salts
are
of
containing
reactive
very
will
phenylamines
react
-Net
an
with
ntuc()
group
and
CHNO2)
at
a
temperature
below
100
salts
dazanium
since
SALT
that
(-Nte)
amine
form
DIAZONIUM
and
is
unstable,
has
it
be
to
made
the
in
sodium
nitrite
(NaNO2)
Hall
so
are
f
NaNOz
unstable
that
they
will
I CI
upon
>
further
HNOz
nitrous
warming
HNOz
+
H91
below
10
NacI
#
N
&
with
acid
NI C1-
NHz
phenylamine
mode by reacting
regress
·lazoniom
sall
#
benzene dlazonium
chlande
2
O
water
to
form
a
phenol
and
Relative
·
and
ammonia
·
the
electron-donating
become
MORE
of
the
COMPARING
order
BASICITY
MORE
BASIC
of
of
pair
dative
for
dative
electrons
·
trend
ethylamine,
in
available
more
·
into
be
the
to
in
as
lane
pair
phenylamine
a
result,
on
ammonia,
electron-donating
the
the
the
lone
lone
a
form
dative
a
&
pair
pair
is
of
aromatic
ring
phenylamine
on
the
(i.e.
benzene
as
follows:
>
group
not
is
electrons
of
pair
and
atom
nitrogen
is
cause
the
lone
of
pair
ring)
the
causes
lone
pair
electrons
of
to
phenylamine
ammoniax
base
at
covalent
of
density
electron
lone
PHENYLAMINE
and
ethylamine
alkyl
dative
nitrogen
their
available
banding
GTISYLAMINE
explained by looking
form
to
banding
an
weakest
the
groups
donates
bond
it
attatched
a
is
to
density
electron
with
ammacksmedatinghence
the
·
can
the
covalent
ethylamine
this
electrons
of
pair
Phenylamine
3
BASK
strongest
·
readily
INCREASE
covalent
LESS
AMMONID,
basicity of
of
for
becomes
amine
their
donate
can
how
on
groups)
alkyl
AVAILABLE
lone
LESS AVAILABLE
the
(i.e.
becomes
amine
delocalisation
the
they
as
lone
proton
a
groups
become
to
the
·
with
bases
as
Ageors Ammonia, Ethylamine
of
basiclyinthemones depend
electrons
·
act
amines
bond
covalent
·
Basicity
to
base
the
the
(-N1z)
amine
nitrogen
group
atom
causing
lone
its
pair
to
become
proton
Less
BASIC
than
however
ethylamine
it
MORE
is
BASIC
than
phenylamine
as
delocalised
electrons
electrons
overlap
become
with
less
the
conjugated
readily
available
system
to
form
on
a
the
band
benzene
with
a
ring
proton
and
becomes
DELOCALISED;
motokist
->
Itnhe
Ethylamine
CH CH2
>
Positive
WHe
inductive
the
to
effect
causing
its
alkyl
lone
donates
group
pair
of
electron
electrons
to
density
become
available
more
Ammonia
N
⑪
-
no
electron
donating
manas
Phenylamine
>With
nitrogen's
benzene
dative
lone
ring
covalent
groups
to
cause
positive
inductive
effect
andana.
of
pair
and
band
is
electrons
therefore
with
15t
becomes
less
relocalised
available
to
in
form
the
a
ooks.
**p
·
Azo
Azo
·
(or dazonium
used
often
compounds:
dyes and
as
compounds
organic
formed
are
compounds
coupling
a
in
have
that
R-N/N-Re
an
between
reaction
dazonium
the
·
a zo
compounds
making
·
formation
4:
Step
NaNOz
reaction
coupling
benzenediazonium
a
of
solution
case
its
of
sodium
of
has
so
be
to
made
in
HCI
test-tube
a
because
nitrors
phenylamine
-
REACTION
-
If
the
given
ON
mixture
salt
with
alkaline
phenol
and
nitrate (NaNoz)
sodium
using
or
with
potassium
nitute
is
made
always
phenylamine, the phenylamine
added.
is
The
is
between
reaction
the
and
is
with
reacts
a
+
cag>
and,
weak
nitrous
+
aco
in
first
reaction
dissolved
and
mixture
in
hydrochloric and
hydrochloric and,
and
the
nitrite
and
cons
then
a
produces
the
HNO2 cage
NO2caq
the
Nack
↳
HNOz
>
readily and
very
reaction
16
-
chloride
acid
nitrous
phenol
PROCESS
&
decomposes
acid
the
In
-
of
NaOI6
IN
nitrous
nitrous
solution
acid (HC)
hydrochloric
-
alkaline
acid
unstable
very
PRENOL
WITH
MULTI-STEP
Nitrous
is
the
from
a
is
of
acid
nitrous
formed
dye
azo
an
be
can
CHLORIDE
an
group
az0
BENZENEDIDIONIUM
and
OH
NN
of
ion
Rz
RI
COUPLING
group
position
equilibrium
of
differently
depending
black
product
on
the
the
right
phenol (amongst
other
lies
well
temperature
WARMING
is
warmed,
you
get
a
only
which
contains
off
C. HsNHz
+
HNOz
>
CgHs
01
+
10
+
Ne
things), and
nitrogen
gas
is
The
reaction
solution
the
-
-
sodium
the
the
-
end
phenylamine
potassium
or
hydrochloric
in
nitute
the
of
above
you
temperatures
low
of
solution
goes
-
at
nitrite
is
then
added
with
up
positive
solution
a
containing
containing
ion,
equation:
lonic
·
Reaction
·
-
the
to
dilute
-
backer
a
of
phenylammonium solution,
benzenediazonum
the
that
so
temperature
the
-N2
1NO2
+
CIchlonde
group,
is
16+
and
nitrous
between
known
as
dazonium
a
N
&
and
con
21628
+
phenylamine
to
the
form
dazonium
ion
called
is
diazotization
Conditions:
reaction
mixture
and
benzene
must
be
kept
100
below
using
ice,
otherwise
the
dazonium
on
thermally decompose
will
nitrogen (N2)
and (i.e. H(l)
N
-N
16N0z
+
↑
HC
2)
2He O
&)
&
phenylamine
benzenedazonUM
chlonde
·
·
the
3:
-
Coupling
dazonium
Reaction
never
chloride:
NH2
Step
ke
Dlazotization
2:
reaction
the
in
NH2
+
Step
stood
is
ke
the
to
solution)
chloride
so a
benzenedlazonium
the
in
slowly
very
IN
-
(phenylammonium
cooled
also
solution
is
and
ion
Reaction
acts
as
an
electrophile
and
substitutes
into
the
benzene
ring
of
the
phenol
at
the
4th
Conditions:
alkaline
conditions
are
required
to
deprotonate
the
product and
organic
form
920
S
NN
C1-
benzened,azonium
chlonde
Old-
HO
↑
phenol
N
N
3
azo
dye
the
azo
compound
group
ON
16 DI
↑
position
delocalised
the
·
-N-N -which
the
·
as
a
MAKING
other
·
·
e.g.
electrons
result
of
OTHER
AZO
eyes
the
eye
yellow
instead
acts
as
bonding
budge
a
delocalisation
the
can
the
in
between
the
two
throughout
electrons
of
systems
the
of
the
rings
compound,
compounds
a zo
followed
be
similar
a
via
formed
be
can
NN
stable
very
are
the
from
route
as
between
reaction
coupling
above
described
benzenedazonium
↳
C1-
Naold
N
↓I Gzo
T
and
chloride
C, HsNCC4g(c
group
City
Reactions
REACTIONS
Cly
-
dlazonium
they
-the
contain
-Not
-the
substitution
-
-
an
group
nitrogen
-wa r m
ions
-
by
an
nitrogen
gas
is
group.
the
dazonium
this
IONs
such
case
as
of
chloride
benzenediazonium
benzenedazonium
WN
CIT
benzene diazonium
chloude
chlande,
solution.
this
is
attatched
to
a
benzene
ring
else
gas
group
chlonde
with
water
in
the
solution
and
phenol
is
formed
-
either
in
solution
or
as
black
evolved
N
-
In
nitrogen
as
Ob
reacts
solutions
in
replaced by something
released
is
on
Net
is
benzene diczanium
diazonium
present
are
Dlazonium Salts
of
DIAZONIUM
of
16CI
&
N
163 C
-
through
DYES
He C
SUBSTITUTION
extended
a re
rings
phenol
of
benzene
two
ion
is
t
formed
H20
and
then
immediately
reacts
+
o
3
with
water
in
the
N2
solution
At
+
to
give
phenol
only 1,qud
substitution
-
add
by
droplets
only
Olzonium
potassium
REACTIONS
lodide
long:
·
·
the
In
w/
Reaction
phenal
a
No0l
in
and
the
used
nitrogen
to
make
bridge
a
dazonum
the
in
between
produce
to
solution
a
-
there
is
product
cooled
is
a
&
->
-
-
-
-
-
-
It
NaOH
the
the
an
N
off
an
has
reaction
is
intense
is
cold
done
is
cooled
orange-red
benzenediazonium
benzene
given
off,
you
Nz
+
rings
C2
under
the
linked
chlonde
phenoxide
by
exactly
a
mixed
precipitate
with
the
the
formed
is
ion
is
and
a
added
yellow
orange
solution
or
pet
ON
N
N
rather
molecule
same
conditions
hydroxide
solution
benzenedazonium
-
another
azo
as
to
than
with
to
a
simple
benzene
ring.
phenol
produce
chloride
solution
compound
OH
S
is
bridge
nitrogen
beta-napthol)
or
O-
N
solution
together
sodium
in
1628
phenoxide
3)
napthalene
a
fused
rings
dissolved
and
are
rigs
napthol
to
phenoxide
Nat
+
8-
attatched
benzene
and
ion
↓
group
two
napthalen-2-01
solution
benzene
3
dazonium
the
napthalen-2.01
w/
napthalene
the
and
compounds: two
azo
contains
lae,
in
between
recation
-N
Reaction
gas
last
is
on
two
sodium
of
sodium
-
nitrogen
IONS
solution
+
solution
I
>
O If
-
cold,
phenol
dissolved
is
retained
is
the
in
#
above,
reactions
nitrogen
coupling,
for
-
substitution
solution
-
t
DIAZONIUM
of
chlonde
formed
N
COUPLING
benzenediazonium
the
to
solution
codobenzene
of
+
ions
atom
lodide
potassium
get
-
iodine
an
NN-
an
on
just
like
the
phenal
one
formed
liquid
some
-
w/
reaction
The
phenylamine (amline)
phenylamine
added
is
to
cold
a
solution
chlonde, and
benzenediazonium
of
the
mixture
is
shaken
vigorously
solid
yellow
a
-
produced
is
NN
-
the
-
-
-
made
one
compounds
azo
-
white
If
-the
the
-
light
colour
groups
of
you
-
-
the
is
result
groups
present
an
of
azo
N
O, S
this
"an line
yellow"
eyes
orange
system
which
electrons
of
take
some
delocalisation
molecule
wavelengths
benzene
both
in
and
rings
the
two
rings
well
as
absorbed
are
by
delocalised
these
electrons
wavelengths
(and
have
can
benzene
the
to
non-absorbed
the
the
the
in
attatched
molecules,
of
to
the
so
effect
absorption
on
the
light
on
the
pat
of
light)
known
are
absorbed, and
so
the
on
as
a
color
chromosphere
see
you
indicators
in
dye
dyes.
az0
modern
things
to
these
of
contribute
is
orange
one
as
rings
extend
which
advantage
take
can
methyl
-
the
the
as
of
highly relocalised
a
on
see
you
modifying
-
falls
half
indicator-methyl
an
also
can
known
is
than
more
budging
delocalisation
the
as
contain
atoms
nitrogen
-
dye
for
used
frequently
are
16+
+
NAz
N
N
3
(aniline)
phenylamine
account
azo
of
use
compounds
azo
from
compounds
azo
The
coloured
strongly
these
NHz
t
which
exists
N
two
in
(CH3)
N
depending
forms
red
form
methyl
of
orange
2
H
-
-
as
the
and
-when
-
-
you
methyl
If
In
add
add
and
is
at
red
is
some
lost
is
cons
yellow
paint
there
a
p1
will
removed
>
of
hydrogen
solutions (in
are
at
there
wavelength
orange,
acidic
in
yellow
2
gained
or
the
in
methyl
It
(CH3)
N
shift
to
alkal.
orange
between,
ion
a
causes
orange
you
-methyl
-
hydrogen
that
N
N
O, S
fact
and
is
a
shift
ion
attatches
solutions
you
the
in
absorbed.
light
of
get
of
form
exact
Obviously
to
give
plo
the
methyl
orange
nature
that
the
of
means
red
the
delocalisation
you
see
a
in
different
form
< 3.
yellow
from
4.4
be
equal
amounts
of
red
and
yellow
forms
so
methy/orange
looks
orange
the
molecule,
colour
Reactions
·
Amides:
·
the
formed
amide
(CONR)
group
with
hydrolysis
reduction
HYDROLYSIS
condensation
from
with
this
·
link
be
broken
amides
by
links
hydrolysis
with
ammonia
or
amines
including:
reactions
hydrocarbons
two
by refluxing
amide
it
sections
with
and
an
molecules
their
of
together
alkali
or
a re :
aco
a
>
C
R
undergo
can
chlorides
acyl
or
amd
non-substituted
a
of
carboxylic
ammon,
or
substituted
in
can
products
the
·
mali
compounds
acids
AMIDES
Of
amide
these
in
carboxylic
of
y
the-CON-group
·
reaction
Amides
of
R
C
N163
t
Old
N-$
Carboxylic
ammon,
a
acid
Id
products
·the
substituted
of
a re :
acid
carboxylic
primary
amide
amine
R--N-R
>R-c
·
will
ammonia
react
and
carboxylic
will
t
NHe
olt
is
·
"
in
get
excess
and
to
form
deprotonated
in
in
dilute
R
primary
carboxylic acid
an
exc e ss
ammonium
base
to
amine
salt
form
a
carboxylate
con
Hydrolysis:
reaction
When
the
The
with
amides
amide
and
alkaline
water.
are
hydrolysed
presence
of
cads
Ge169).
The
and
cats
as
a
catalyst
for
the
reaction
between
water
hydrolysis
of
amides
involve
reaction
with
OH-ions, but
the
result
is
similar
enough
it
is
classified
as
hydrolysis
16
I
is-N -16
I
amman, UM
sald
19
the
for
drying
changed
is
Cy CON1,
e.g.
and
water
+
produce
reacting
with
CsCook
-
R
anot
and
<CL
&
ammonium
etheroate
C
+
Fi
N1nCI
16
R
N
A
>
would
and
R
would
which
also
is
catalyst
a
as
s
R
1620
16 +
Oil
It
acts
I
cod
ethanoic
make
to
but
ethanoatesons).
and
lons
still
reaction,
amide
between
reac tion
ethanoate Sammonium
lons
the
A
contain
ethanoi
solution
of
ammonium
chloude
aad
-
N-$
Id
↑
alkaline
CNCOH]
aOH) R
8Carboxylate
e.g.
If
-
you
and
you
then
+
hydrolysis
test
alkaline
Using
-
CI, CoNH2
it
can
is
Nook
an
to
an
to
Ncold
for
unknown
an
Cl,
>
the
ammanca
by
-
in
gaseous
state
COON
a
+
Noy(3)
amide
organic
smell
ammonia
con
compound,
and
it
gives
off
ammonia
amide
recognise
R-N1 k
+
C
because
it
turns
red
litmus
paper
blue
on
heat
(not
immediately
in
the
colds,
REDUCTION
the
·
c-0
group
products
the
·
amides
in
be
can
non-substituted
a
of
primary
a
H.
AMIDEs
OF
amine
-CONs
and
amide
reducing
strong
LiAlty
to
Recsents
1
Lifly
dry
agent
asent
reducing
CLiDilu
St
"ase
primary
1620
&
-
in
a
substituted
a
of
secondary
and
amide
amide
Initial
b
DEISYDRATION
·
·
·
·
amides
are
water
the
↓
with
e.g.
nitrile
is
·
amides
room
·
the
can
<-c-N.-x+128
CIs ]
be
a
cod
Recsents
1
viply
dry
in
conditions:
ether
at
room
temp
-
Initial
reaction
treatement
mome
with
is
dilute
followed
by
cod
AMIDES
the
from
collected
you
OF
amide
mixture
leave
a
the
of
nitrile
group
amide
-
and
phosphorous
Pa0o
CN
distillation
>
12 g
Ch3
CN
AMIDES
reduced
reaction
(v) oxide,
exhamentaile
PO,0
-
to
group
by simple
will get
solid
a
to
primary
amines
by
reaction
with
lithium
tetrahyondoaluminate,
LiAlhy,
temperature
initial
-
is
is
20
ethanamide,
REDUCTION
by
followed
is
dilute
with
Id
15
i
CHs CONHz
THE
4
dehydrated by heating
removed
is
liquid
OF
room
are:
cnide
20
at
water
If
#
ether
reaction
LiDiky
@N-3-s
conditions:
amine
-
products
the
amine
temp
treatement
·
an
form
a re :
cwide
primary
the
by
water
Of
t
REDUCED
is
followed
by
treatment
CHeCONIe +UOentirely
with
dilute
~
add
I.e.
Cly C12 Nic
Hasty
+
or
162 @
HC
in
dry
ether
Cethoxyethane)
at
Relative Basicity
·
·
Base:
a
amines
a re
species
·
the
(such
BASIC
as
basicity
nitrogen
lone
its
has
atom
of
pair
electrons
to
form
humpamentions
a
which
COVALENT BOND
DATIVE
a
can
form
Amines
dative
a
covalent
another
with
band
with
species
electron-deficient
an
16+con)
the
MORE
the
donate
can
the
as
an
of
the
·
that
species
Amides and
of
LESS
ELECTRON-DONATING
depends
amine
READILY
on
such
GROUPS
as
availability
the
AVAILABLE
lone
lone
the
AVAILABLE
READILY
the
alkyl
pair
pair
groups
this
of
lone
electrons
of
electrons
of
increase
pair
is,
dative
for
is
bonding,
covalent
WEAKER
the
electron
the
electrons
of
density
BASE
BASE
THE
on
STRONGER THE
the
the
nitrogen
atom
causing
the
mmptobee
e
or
available
nation
·
GROUPS
ELECTRON-WITHDRAWING
such
aromatic
as
benzene
wings,
delocalisation
cause
of
lone
the
pair
becomes
which
electrons
of
and
lessrcalyavable
·this
an
is
why
electron-donating
B
ASICITY
·
·
·
amides
to
of
also
the
contains
an
electron-withdrawing
benuene
ring)
a
is
WEAKER BASE
than
propylamine (which
contains
groups)
alkyl
AMIDES
contain
NITROGEN
a
basicity
the
again,
One
PIENYLAMING (which
presence
of
of
ATOM
amide
the
with
depends
a
lone
on
the
electron-withdrawing
the
electrons
of
pair
availability
oxygen
atom
of
the
the
in
lone
amide
pair
for
dative
density
electron
group,
bonding
covalent
is
removed
from
the
nitrogen
atom
·
the
lone
pair
on
the
nitrogen
atom,
therefore, become
less
readily
available
and
not
is
available
to
donate
to
an
electron-deficient
species.
·
since
amines
this
electron-withdrawing
oxygen
is
characteristic
of
amides
and
is
not
present
in
amines,
amides
are
much
weaker
bases
than
T HE
LACK
Unusually
-
THE
compounds containing
primary
-
the
-
with
compound
a
has
dosen't
It
source
like
make
-
methylamine,
huge
a
words
other
in
by
picks
ammonia
on
lone
has
as
a
hydrocarbon
a
ammonia
in
[ChaNAa)
amime
weak
bases
group
combine
can
are
with
hydrogen
a
ion
(a
proton)
base
happened
that
is
one
of
the
hydrogen
atoms
attatched
to
the
the
to
lone
and
pair
so
methylamine
and
ammonia
behave
similarly
hydrogen
a
up
atom
to
primary
a
or
group
difference
of
neutral
a re
NA,
attached
is
acts
that
all
ammonia,
nitrogen
it
methyl
a
amount
the
amides
GROUP
group
on
group,
Ie
group
-Nke
electrons
replaced
been
Nic
where
of
pair
other
some
nitrogen
-
lone
-
-N1dz
THE-N12
OF
an
compound
amine:
active
from
-
CHARACTER
BASIC
AMIDES
IN
the
containing
compounds
for
USAL
simple
-
BASt CHARACTER
of
it to the
by attatching
the
nitrogen
pair
·At
on
N.It
16:
S
N.It
16:
&
o
·
⑧B
H
wouldn't
i
If
this
cly
molecule
was
equilibria
NHscags
-
-
-
novice
in
these
both
turn
that
both
cases
compounds
ammonia
red
reactions
the
the
and
litmus
weak
the
blue
like
amines
these
step
are
lone
nitrogen
takes
pair
a
hydrogen
on
from
a
water
up:
NHicaps
+
CH, N16, "cag>
OH
+
cap,
01-cage
reversible
of
bases
amines
water, the
in
1620ck
+
are
positions
are
and
H20ci
+
CHy N11zcaq)
-
a
ammonia
compounds
these
and
-
difference
replaced by
much
similarly
dissolve
you
If
hydrogen
group-so
behave
e.g.
make
are
equilibrium
he
because
they
alkaline
in
well
to
don't
solution
the
hang
left.
on
because
to the
of
incoming
the
hydrogen
presence
of
the
con
very
hydroxide
well
cons,
and
both
of
them
WHY
-
-
-
-
amides
their
tendency
need
we
like
any
other
double
electron
band
~
amide,
an
form
to
look
band,
the
found
lone
on
pair
and
above
the
line
the
band
lone
the
overlaps
and
result
on
·
and
oxygen
this
purposes
nuclei
the
carbon
and
almost
parallel
on
of
to
parts:
bond
a
->
the
these
aband
the
in
oxygen
and
porbitals,
them
with
overlaps
as
they
two
the
lone
anything
delocalisation
in
pair
makes
delocalisation
harbon
out
them
the
of
hydrogens
on
way)
the
of
nitrogen,
the
is
as
nitrogen
prevent
no
longer
attractive
molecules
would
have
lone
electrons
the
but
which
like
positions
delocalised
the
atom,
effects
(the
pielectrons
that
is
nitrogen
because
this
involved
pibonding
the
isn't
·
-N1c group
nitrogen
the
on
with the
taking
of
has
this
pair
gets
carbon
-
the
pibond
the
found
most
for
different
a
of
molecule
goodjaman
the
ignored
the
plane
up
up
high of
-
all-despite having
at
be
can
two
the
porbitals
ends
it
made
is
the
atom
character
group
between
below
between
nitrogen
that
-CoNte
double
basic
no
slight
so
the
AMIDES?
WITH
virtually
is
in
on
overlap
sideways
-
cons
carbon-oxygen
found
is
pair
have
bonding
a
is
pair
and
hydrogen
the
at
HAPPEN
SIMILAR
litmus
to
attract
to
electron
other
In
neutral
are
one
-
SOMETHING
DOSENST
more
to
the
from
lone
located
for
a
stable
be
becomes
pair
it
single
a
broken,
the
nitrogen
and
that
out
to
will
as
-
over
hydrogen
atom
hydrogen
nearby
for
spread
accepting
pair
on
are
delocalised
an
the
cons
other
in
whole
and
intensely
words
part
acting
it
of
that
a
as
is
no
longer
molecule
base:
negative
region
and
to
of
space,
it
con
reclaim
cost
its
lone
energy
pair
join
a
hydrogen
on,
Acids
Amino
Add/Base
·
Properties
Acids:
Amino
basic
a
·
One
to
the
presence
they
NATURALLY
·
·
·
2-amino
carboxylic
next
the
to
these
there
type
are
-cool
of
28
amino
both
of
that
B
functional
two
Iselectric
the
Paint
groups
group
group
acidic
and
basic
a
Zwitterions
contain
acid (-coot)
carboxylic
in
group
amino
acids,
they
are
said
to
AMPDOTERIC
be
teen
memes
board
can
OccURING
(-NHe)
amino
acidic
an
compounds
organic
are
Acids,
Amino
of
AMINO
acids
ACIDs
type
a
are
of
acid
amino
which
in
ame(-N1z)
the
group
is
bonded
to
the
carbon
atom
group
acids
naturally
the
from
occuring
amne
building
acids
amino
that
blocks
with
the
make
99:
up
proteins
RCH(N1)
2004
NIz
->
group
R
C
$
cool
->
carboxylic acid
group
amino
↑
R
grarp-acdic
100C
add (RCI6 (N16) CooK)
NIz
NIz
S
CH2
C
$
200l
aspartic
acid
R
group:
160
basic
CH2
R
C
$
200l
group-neutral
NIz
To
C16
3
C
$
200l
senne
alanine
PROPERTIES
ACID/ BASE
·
acids
amino
undergo
will
reactions
most
with
amines
·
however, they
can
2witter
on
because
·
son:
with
changes
of these
acids
amino
zuitterion,
and
are
therefore
there
acids
s
a
(within
themselves)
negative
(-coo-]
charge
intermolecular
forces
strong
are
soluble, crystalline
"NAz
Rate
↳
intermolecularly
a
in
carboxylic
acid-base
including
reactions
of
bases
positive [-NHy")
a
and
amines
of
with
acids
interact
also
AcDs
acids
carboxylic
·
AMNO
OF
to
a
form
of
ZWITTERION
between
attraction
amino
acids
solids
amphoten
J
>R-C -16
doo
cold
zwitter on
ISOELECTRIC
·
a
solution
they
·
act
POINT
acids
amino
of
BUffER
as
will
water
in
SOLUTIONS
exist
IWITTERIONS
as
they
as
resist
any
with
changes
both
in
acidic
and
when
small
pH
basic
added
·
If
an
acid
is
-coo-part
the
this
If
a
base
the
this
the
causes
INCREASE
·
added (and
IN
is
-NH
causes
ACID
of
the
thus
the
pH
will
criterion
zuitterion
to
CLOWERING
+
the
the
zwitterion
coo&
witter,
or
to
become
donate
will
a
an
H
the
cool
group
con
Aftian
negatively charged
-NH3
+
reform
RAISEDC:
is
↑NH3
#3-16
Icon
positively charged
a
pH
zuitterion
to
an
pH)
thus the
of
accept
become
added (and
part
LOWERED):
is
~
R)
-
16
coolt
positively
charged,
on
on
to
reform
the
-NIdz
group
amounts
properties
of
and
or
alkali
are
INCREASING
·
If
a
base
the
this
is
-NH
added (and
thus the
+
the
part
the
causes
pH)
BASECRAISING
IN
of
zwitterion
pH
zuitterion
become
to
RAISEDC:
is
donate
will
a
016
+
~
-
the
lons
pit
be
can
donate
this
negatively
slightly adjusted
is
the
amino
called
to
acid
the
ISOELECTRIC
plo
↑NH3
lon
a
as
charged
point
a
POINT
NEUTRAL
on
16
charged
↓
on
neither
which
at
the
R)
-
NHe
16
zWitten
Old-
816-
+
cooneutral
positively charged
High po
-
+
or
acid
point
16+
I6
negatively charged
the
[WITTERION
amino
↑NH3
2001
positively
group
428
selectric
-
-
reach
exists
Low
R)
-NIdz
coo-
or
and
the
reform
on
+
R-C-16
coo-
·
to
NHz
#3-16
witter,
Aftian
an
negatively charged
↑NH3
&
·
R)
-
16
cooon
negatively charged
on
ACID-BASE
-
an
BEHAVIOUR
and
amino
both
has
basic
a
ACIDS
AMNO
Of
and
group
amine
acidic
an
carboxylic and
group
basic group
wide
Red-coold
Tacoic
-
there
with
internal
an
is
both
hydrogen
a
of
and
charge
negative
a
transfer
charge
positive
a
the
from
ion
group
-cool
the
to
-Nic
group
leave
to
an
on
ION
IWITTER
->
group
-itscoo
&
-
this
the
is
that
form
solution
simple
&witterion:
compound
If
is
alkali
an
to
removed
pro
of
N16,+
in
simplest
its
crocodile
-
-
a
of
although
the
-
drop
clip
the
paper
amino
at
amino
the
is
and
overall
no
-NHy-
the
from
Rcs
-
sold
the
in
state.
If
you
dissolve
the
amino
and
in
water,
a
cor
charge,
electrical
but
which
contains
separate
parts
which
positively and
are
acid
amino
allowed
would
end
to
be
solution
of
attatched
is
dry
to
placed
colourless,
and
found
by adding hydroxide
>2 M coo-+
Old
electropherosis
is
and
amino
an
ions,
the
hydrogen
lon
group.
#
solution
add
the
-coon
form,
each
acid
amino
an
the
increase
you
with
even
in
con
charged.
negatively
Adding
exist
this
contains
also
a
is
adds
amino
witter
then
to
just
can
consist
of
a
piece
1620
mastened
of
filter
paper
on
a
with
a
microscope
slide
with
a
battery.
a
in
its
the
position
heated
travel
centre
of
after
gently,
towards
the
a
the
paper
a
time
amino
anode (the
can
and
be found
shows
positive
up
by
as
electrodes
spraying
a
it
coloured
spot.
solution
of
ninhydrin.
If
Adding
decrease
you
If
-
add
an
to
the
and
amino
an
pit by adding
add
an
solution
to
solution
a
of
and,
amino
an
the
part
-coot
of
the
zwitterion
picks
up
a
hydrogen
1OU
N
g
t
R-dis-coo
Haq
+
i
NPscoold
notice
↑
-
the
shifting
-
-
suppose
that
the
-
the
p
start
you
contains
you
the
with
acdi
two
extreme
one
from
hydrogens
-
the
positive
a
now
lon
zuitterion
a
the
under
could
addic
and
group
and
conditions
that
so
is
and
slowly
removed
alkali
it.
to
first-and
back
get
you
to
zwitterion
addic
more
these
of
the
is
the
in
one
Redscook
-so
in
one
longer
is
other
produced
just
we've
son
the
to
no
that this
when
added
have
you
group,
and
so
that
is
removed first-
and
back to the
get
you
cutterior
N16,
>R-d -c00t+
015-
t
amount
right
the
just
cools
of
alkali, the
amino
and
no
longer
128
has
a
net
positive
or
negative
charge.
-
-
that
the
a
-
-
means
pit
at
wouldn't
that it
which
lack
this
towards
move
of
during
movement
either
the
cathode
or
electropherosis happens
anode
is
during electropherosis
known
as
the
selectric
point
of
the
amino
cid
the
If
ply
you
hydrogen
va n e s
go
ion
from
to
adding hydroxide
on
is
and
amino
removed
from
N16,
Rdx
ions,
-N1st
ac
you
will
get
the
reaction
016
already
we've
group
NAz
+
-coo
the
amino
R- -coo-
&
162 8
seen,
in
which
a
-
that
-the
-
by
process
adding
contains
con
-N12
that
leads
the
back
you
basic
two
is
group
groups-the-N1c
base, and
stronger
the
to
cutter
picks
so
-....
keep
can
you
16
↑
-coo-
by
going
the
and
group
up
Why
isn't
when
the
adding
then
hydrogen,
a
as
and
at
this
critterion
paint
dissolves
in
the
water,
and
amino
the
-as
situation
Ny
-
the
with
interacts
it
is
group
a
is
when
bit
little
complicated
more
that
we
tend
water
molecules
acting
-
as
both
add
an
and
base
a
NIz
only
a
weak
a
is
and,
week
and
R-coo -+
and
the
donates
position
Yis"-coo-
coo-group
again,
-c00-group.
p<?
of
a
hydrogen
equibrium
ion
will
be
base:
a
the
to
level.
R
-
finished
acid:
an
because
-
just
first.
cons
at
NI6s
-
we've
2_Nscook
~
an
of
on
R-s-coo-+1620
-
low
>R-scoo
"aa,
Hags
+
selectric
the
amino
an
pretend
-
the
-coo-group.
hydrogen
N16z
R-60-c00.
-
to
con
NI62
R-cx
-
and
an
with
up
-
whole
the
reverse
of course,
can,
you
the
you
is
equilibrium
dissolve
a
lies
an
weak
to
amno
16. 8
base
the
2
and
takes
a
to
to
water
a
1698
molecule
left
the
ms "cool
hydrogen
ion
from
to
a
water
molecule
left
said
in
water,
both
of
these
reactions
a re
happening
to
But...
-
-
-
the
positions
e.g.
simple
that
the
-
&
-
positive
rift
to
of
you
can
equilibrium
-
-
amino
are
do
e.g.
for
you
be
will
of
rather
they
equilibrium
first
more
-
of
lies
negative
the
depending
vary
bit
a
on
further
from
the
to
on
the
amin o
the
influence
right
and
in
the
of
R-group
second
than
the
the
solution
one
than
the
you
if
carried
out
the
positive
towards
acids
need
to
down
cut
electropherosis
on
the
unmodified
would
solution, there
be
slight
a
electrode (anodes
the
amount
of
the
negative
so
can
that
concentrations
the
of
the
identical.
by adding
that
further
typically,
position
->
identical
aren't
one
that,
cons
equilibria
two
there
circumstances,
stop
two
-
that
the
acids
amino
means
those
In
of
the
to
the
glycine,
clanme
serine
->
to
the
->
p1
very
small
amount
of
and
to
the
solution,
moving
the
position
left
has
old
a
be
lovered
selectric
p16.11
3.sg
to
paint
about
is
6
to
p165. OT
achieve
3
this
pants
selectic
there
cool
is
an
group
a re
additional
different
Nic
group
when
or
of
the
first
Bonds
Peptive
formation
each
·
the
·
-Ntz
the
one
of
an
and
(-Nide)
amine
and
amino
is
react
can
condensation
between
condensation
a
Ospeptide
the
bond
amide
new
this
since
·
group
contains
aco (-cool)
carboxylic
the
with
-cool
group
of
group
another
add
amino
CONDENSATION
a
in
REACTION
to
form
DIPOLE
a
·
and
amino
Bonds
Peptide
of
still
contains
reaction
to
two
reaction,
an
-
NA,
small
a
and
-cool
called
is
molecule
condensation
reaction
-
R
acids
this
(in
case
each
at
group
PEPTIDE
a
H20)
end
N
C
If
amino
N
C
0-1
If
and
BOND
ELIMINATED
is
of
PEPTID
or
molecule
the
which
*
16
C
0-1
If
I
aCO
aMIM 8
N
C
If
16
N
C
If
C
N-a
O
i
If
a
POLYPEPTIDE
Id
Id
If
is
formed
when
N
If
C
amino
C
N
S
0-1
If
Il
Id
16
join
together
"ONNE
·
N
acds
U
polypeptide
N
O
S
O
tapeptide
many
a
cc-N-CO-N-ce CoR
A
dpeptide
·
in
bonds
*
16
0-A
participate
Opeptide
2
↓
R
C
If
peptide
R
again
R
C
16
can
peptiveand
R
16
16
LINK
TRIPEPTIDE
a
form
amino
to
form
a
long
chain
of
molecules
0-A
Electropherosis
Electropherosis:
·
method
this
·
·
a
the
sample
positively
the
negatively
fast)
the
·
Electropherogram:
CACI
·
·
·
the
charge
the
movement
consider
a
BOND
of
sample
amino
amino
the
cons
which
and
A:
add
B:
and
H-
adds
glycine,
-clon
Wis
amino
bands
depends
acid
which
By
on
the
pit
of
a
side-chain
side
to
a
quickly
more
paper
or
gel
electrophoresis has
after
metic
are
pros
solution
amino
acids
will
at
therefore
plo
be
affected
by
the
plo
(
positively charged
is
is
chain
neutral
is
charged
negatively
H-N-<4-co
amino
A
on:
pId
the
three
of
proteins
ELECTRODE
depends
the
on
during electrophoresis
mixture
side-chain
THE
of
move
cons
corresponds
VARYING
field
slowly
observed
are
purify
electrical
an
ELECTRODE
Positive
the
and
in
electrodes
NEGATIVE
electrodes
more
charged
electrodes
the
glotamic and,
[cita)a
highly
HCIDS
consists
lysine,
:
N'-c4
to
move
cons
electropherogram
the
AMINO
of
amino
on
amino
in
cons:
the
towards
move
the
towards
move
charged
them
placing
identity
to
oppositely
towards
move
will
long
larger
of
series
will
lons
cons:
the
of
the
MIXTURES
SEPERATING
the
charge
the
which
two
between
by
sons
analysis
brochemical
in
cons
charged
at
of
size
placed
is
charged
the
rate (how
used
separates
which
technique
often
is
acids
amino
of
analytical
an
ac
H-
N'-c4
-clon
[cita)a
B
o
o-
amino
and
c
occured
·
the
amino
acids
amino
amino
amino
·
since
to
this
in
and
and
and
I
A
glutamic and
will
will
B
will
is
mixture
move
remain
in
move
towards
LARGER
than
separated by electrophoresis
be
can
towards
the
well
the
lysine,
where
negative
it
positive
the
will
the
electrode
sample
applied
is
the
to
gel
electrode
towards
travel
the
positive
electrode
electrode
lysine
↑
amino
wel
amino
amino
-
electrode
acid
acid
(
B
and At
at
a
SLOWER
RAT
compared
formation
·
Polymerisation:
Addition
made
to
·
Condensation
Polymensation:
using
polymers
form
another
type
a
that
Polyesters
have
double
c-c
bands
joined
together
polyethere
i.e.
used
reaction
of
molecule
small
-
of
monomers
(e.g. H20)
lost
is
the
in
formation
the
when
of
monomers
polyesters
join
together
to
form
a
polyester
contain
polyesters
-
linkages
ester
(
cite
assuredoing
polymer
FORMATION
·
a
dial
a
a
dicarboxylic
a
dial
contains
2-016
acid
dicarboxylic
and
required
are
contains
could
2
yes
↳
are
a re
·
the
polyester
expelled
resulting
as
a
is
water
polymer
is
formed,
molecule
a
to
a
form
polyester
groups
say
lettes
Ethan-1,2-d01
the
H
polyester
a
DcacrobsSophieCatThe
oldgroupsatthesales
2
when
I
groups
D O)
·
for
C
POLYESTERS
OF
and
structure
⑪
so:
-----------
benzene-1,4-dicarboxylic
on e
of
the
old
groups
on
add
the
(1628)
polyester
Ho-cK2-cavityo-a--a-on
O
ethon-1,2-001
Theall
↓ enzene-1,4-dicarboxylic
·
·
↳onofrepealing and
on
aco
diol
and
the
hydrogen
of
the
cool
I
OD
the
HYDROXY
·
·
·
·
so
there
a
the
is
single
these
ACIDS
CARBOXYLIC
far
examples
another
monomers
they
group
making
to
containing
called
a re
contain
making
of
route
manomer
polymer
etchesonI
an
both
group
(-COOK)
2
using
on
separate
for
monomers
the
polymerisation
polyesters
the
of
hydroxycarboxylic
alcohol
focused
have
polyesters
(-oi)
2
key
functional
groups
a
This
be
also
used
acids
at
end
the
of
the
Hydroxybutana add
of
can
hydroxy
monomer
carboxylic acid
contains
is
molecule
an
while
the
other
end
is
eg
monomer.
both
functional
thereeves pope,pois-conte
is
Theall
·
·
·
t
Ps to it
is
i
alsent
polymensing 2-hydroxybutanoic acid
makes
ester
a
links
condensation
polymer containing
O
capped
by
a
carboxylic ac