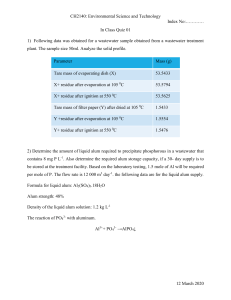
Gugliotti 1 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H,Ryan Gugliotti Section 61 25 October, 2022 Experiment 6: The Synthesis of Alum Lab Abstract - In this experiment, a type of alum called potassium aluminum sulfate dodecahydrate, KAl(SO4)2 • 12H2O, was synthesized. This compound was synthesized by placing the appropriate ions in the same container of aqueous solution, and then evaporating the water to form the alum crystals. Aluminum is considered a reactive metal, which allows for the observation process of synthesis in both acids and bases. Introduction - This experiment is important because it allows for the understanding of how crystals form. The reader should be interested because through this experiment the reader will discover the process and tests of crystal formation. Also, aluminum foil is the limiting reagent in the reaction. The question to be answered is, will alum crystals form through this experiment? - beta-static.fishersci.com/content/dom/fishersci/en_US/documents/programs/educ ation/regulatorydocuments/sds/chemicals/chemicals-h/S25358.pdf Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 2 Experiment 6: The Synthesis of Alum Lab Materials - 250 mL beaker - Aluminum foil - 25 mL graduated cylinder - 3M Sulfuric Acid Solution - Büchner funnel - Baking soda - Watch glass - 3M Potassium Hydroxide Solution - Glass stirring rod - 50% v/v ethanol solution - Ring stand - Vinegar, dilute CH3OOH - Iron ring - Ice bath - Fume hood - Balance - Hot plate - 1.0 L beaker - Tweezers - Filter flask - 5.5 cm filter paper - Beral pipet Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 3 Experiment 6: The Synthesis of Alum Lab Procedure 1. Obtained and wore Proper PPE. 2. Measured 1.00g of aluminum foil, tore into squares ¼ inch by ¼ inch, and added to 250 mL beaker. 3. Performed steps 3-8 in the fume hood. Set up a Büchner funnel and filter flask so that reaction mixture is ready to filter. 4. Slowly added 25 mL of 3M KOH solution to the aluminum pieces. Carefully stirred with the glass stirring rod. 5. Once aluminum had completely reacted, the reaction was filtered using the Büchner funnel and the filter flask. Rinsed the reaction beaker with deionized water. 6. Poured the filtrate solution back into the 250 mL reaction beaker. 7. Once the solution was at room temperature, 35 mL of 3M H 2SO4 was slowly added. It was then stirred with a glass stirring rod due to the foam. Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 4 8. There was a solid remaining after the addition of H2SO4; filtered the mixture using the Büchner funnel and filter flask with a fresh piece of filter paper, then poured filtrate solution back into the 250 mL reaction beaker. Experiment 6: The Synthesis of Alum Lab Procedure (Continued) 9. Gently boiled the mixture until it evaporated down to 30 mL of the solution. 10. Placed beaker in an ice bath for 15 minutes. During this, one area at the bottom of the beaker was scraped to create a nucleation site for crystal growth. 11. Obtained a new piece of filter paper. Obtained and labeled a watch glass. Weighed watch glass and fliter paper together. Collected the alum crystals by filtering them. Washed crystals with 50 mL of aqueous ethanol solution. 12. Filtrate contained solid particles; then obtained a new filter flask. Attached Büchner funnel with some filter paper from step 11. Slowly poured the filtrate from the original filter flask into the existing Büchner funnel with the same filter paper from step 11. Did not wash with the 50 mL of aqueous ethanol solution. 13. Using tweezers, carefully and gently transferred the filter paper and crystals from the Büchner funnel to the previously labeled watch glass. Allow the crystals to Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 5 dry at room temperature for 7 days. After drying, measured and recorded the mass of the sample of alum. Experiment 6: The Synthesis of Alum Lab Collected Data -There was no collected data for this experiment Calculations & Table of Calculated Results Theoretical yield=𝑔𝑟𝑎𝑚𝑠 𝑎𝑙 𝑢𝑠𝑒𝑑 𝑥 𝑚𝑜𝑙 𝑎𝑙 26.98 Answers to Post-Lab Questions 1: 2: 3: 4: 4A:2AL+4OH-+6H20→2[ALOH₄)]-1+3H2 𝑥 1 𝑚𝑜𝑙 𝑎𝑙𝑢𝑚 1 𝑚𝑜𝑙 𝐴𝑙 𝑥 461.73𝑔 𝐴𝑙𝑚 𝑚𝑜𝑙 𝐴𝑙𝑢𝑚 = 𝑔 𝐴𝑙𝑢𝑚 Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 6 4B;Al(OH)3(s) + 3 H2SO4(aq) ---> Al 3+(aq) + 3 HSO41- + 6 H2O 5:A synthesis reaction involves taking reactants and they synthesize or make a substance different than one of the two reactants 6:They appeared sharp but were soft in texture Discussion:Experiment 6 involved the synthesis of alum.Jacob and I had to wait an extremely long time for the water to fully filtrate but it was worth it,we synthesized a large amount of alum and from Experiment 7 we know that the synthesis was accurate and was indeed alum. Conclusion:The synthesis of alum demonstrated multiple precipitate reactions,but ultimately examined the synthesis reaction involving alum.Some errors along the way we could have encountered could have been we added to much water,as aforementioned in discussion of experiment 6 we waited nearly 30 minutes longer than the next lab group for ours to precipitate. Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 7 Experiment 7: The Analysis of Alum Lab Abstract - In this experiment, certain tests were carried out to verify if the compound formed was indeed the compound desired. The compound that was tested was the compound that had been recently synthesized from the previous lab. Three tests were used to determine the melting point, water of hydration, and the percent sulfate of a sample of alum. These tests verified the chemical formula of a sample of alum. Introduction - This experiment is important because it allows for the proof and verification of the alum crystals. The reader should be interested because through this experiment Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 8 the reader will discover the chemical formula of a sample of alum. The question to be answered is, what is the balanced chemical formula of a sample of alum? - beta-static.fishersci.com/content/dom/fishersci/en_US/documents/programs/educ ation/regulatorydocuments/sds/chemicals/chemicals-h/S25358.pdf Experiment 7: The Analysis of Alum Lab Materials Part I, Percent Sulfate Test Materials: Part II, Melting Temperature Test Materials: - 250 mL beaker - Melting point apparatus - Watch glass - Capillary tubes - Balance - Alum crystals - Filter paper - Mortar and pestle - Deionized water - Glass funnel - Alum crystals Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 9 - 0.20 M Barium Nitrate Solution - Hot plate - 50/100 mL graduated cylinder - Glass stirring rod Part III, Water of Hydration Test Materials: - Crucible - Crucible tongs - Balance - Evaporating dish - Bunsen Burner - Striker Experiment 7: The Analysis of Alum Lab Materials (Continued) Part III, Water of Hydration Test Materials: - Paper clip - Iron ring - Ceramic tile - Alum crystals - Clay triangle - Ring stand Procedure Part I Determine the Percent Sulfate of Alum: 1. Obtained and wore Proper PPE. Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 10 2. Added 1.00 g of alum sample to a 250 mL beaker with 50 mL of deionized water. 3. Calculated the volume of the 0.20 M Ba(NO3)2 solution that was needed to completely precipitate the sulfate ions. Measured out twice the volume needed and added it to the beaker. 4. Place a watch glass over the beaker and heat near boiling for fifteen minutes. 5. Pre-weigh filter paper, and label watch glass. Filter mixture through the filter paper with the glass funnel. 6. Placed filter paper and precipitate on a watch glass, and placed it in a cupboard. Measured the mass of the cool and dry precipitate. Experiment 7: The Analysis of Alum Lab Procedure (Continued) Part II Determine the Melting Temperature of Alum: 7. Pushed the open end of a capillary tube into the pile of the alum powder. Packed alum into the capillary tube to a depth of about 1 cm by tapping the tube lightly on the table top. 8. Performed three (3) trials to determine the melting temperature of our alum using the melting point apparatus. Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 11 Part III Determine the Water of Hydration of Alum: 9. Ring, ring stand, and clay triangle were set up over a Bunsen burner. Crucible heated to red hot. Measured mass of cooled crucible, Crucible and cover placed in our evaporating dish. 10. Placed about 2.00 g of alum crystals in the crucible, then measured the mass of the crucible, cover, and alum. 11. Crucible set on an angle on the triangle and placed the cover on. The crucible of the alum was heated until no more vapor was escaping. 12. After they cooled, measured the mass of the crucible, cover, and dry alum. 13. Reheated the crucible and alum sample for 5 minutes, and got mass of the crucible. Experiment 7: The Analysis of Alum Lab Collected Data Table 1: Part I - Percent Sulfate of Alum Measurements Mass of the alum before the reaction (g) 0.9990 Mass of the filter paper and labeled watch 53.7081 glass (g) Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 12 Mass of the filter paper, labeled watch glass, and precipitate (g) 53.7081 Table 2: Part II - Melting Temperature of Alum Measurements Trial 1 Trial 2 Trial 3 Melting Begins Temperature (℃) 90.7 90.7 90.7 Melting Ends Temperature (℃) 93.1 93.1 93.1 Table 3:Determine the Water of Hydration of Alum Mass of crucible and cover 49.5495 Mass of crucible,cover and alum before heating 51.5556 Mass of crucible,cover and alum after first 49.3648 heating Mass of crucible,cover and alum after second heating 49.3648 Mass of crucible,cover and alum after final heating 49.3649 Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 13 Calculations & Table of Calculated Results -There are no calculations to be made for this part of the experiment Answers to Post-Lab Questions 1:From the data we collected,assuming no critical errors,yes our sample is alum. The percent sulfate,water of hydration and melting temperature confirms this. 2:Mass spectrometry would be a great way to measure if this was alum,you could research alums or certain elements wavelengths and measure this way. 3:I wouldn’t be very confident in our data,many many substances can share similar melting points,it is similar to dna in the fact that it can only be used to exclude rather than include certain samples. Gugliotti 2-15 Ryan Gugliotti Experiment 6 & 7 The Synthesis and Analysis of Alum Lab Report Jacob H Section 61 25 October, 2022 14 Discussion:This was an analysis of a composition then analyzing a sample of alum to see if you could use laboratory methods to measure various properties of alum.All our measured values closely match established literature on alum properties and its extensive and intrinsic properties.Our data was subject to errors though.For example when reweighing after heating with the circle and the sample our weight went down.This is likely a systematic error of not waiting long enough to weigh the sample for it too cool causing the heated temperature to affect the weight or possible an error of the scale. Conclusion: In the analysis of the alum lab we demonstrate through the various methodologies employed we were accurately able to analyze our sample we had synthesized in experiment 6.