542542383-Dokumen-tips-Biology-Investigatory-Project-561e79b91f5a0
advertisement

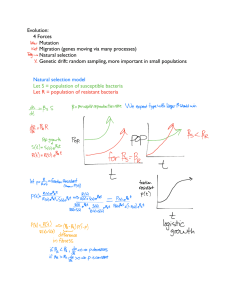
BIOLOGY INVESTIGATORY PROJECT TOPIC: STUDY OF DRUG RESISTANCE IN BACTERIA USING ANTIBIOTICS NAME: ______________________________________ __________ SCHOOL: ______________________________________ ________ ROLL NO: YEAR: 2014-15 INDEX CONTENTS PAGE NO. 1. Certificate of Authenticity 2. Acknowledgement 3. Aim of the project 4. Introduction 5. Need of the Experiment 6. Material Required for the experiment 7. Experimental Procedure 8. Observation and Conclusions 9. References CERTIFIC TE OF UTHENTICITY This is to certify that, __________________________ student of class XII has successfully completed the research project on the topic „„STUDY OF DRUG RESISTANCE IN BACTERIA USING ANTIBIOTICS‟‟ under the guidance of _____________________________ (Biology Teacher). This project is absolutely genuine and does not indulge in plagiarism of any kind. The references taken in making this project have been declared at the end of this Report. Signature (Subject Teacher) Signature (Examiner) NCKNOWLEDGEMENT I would like to express my special thanks of gratitude to our Biology teacher Mr. _________________________ as well as our Principal Mr. ___________________________ who gave me the golden opportunity to do this wonderful project on the topic “STUDY OF DRUG RESISTANCE IN BACTERIA USING ANTIBIOTICS” which also helped me in doing a lot of Research and I came to know about so many new things I am really thankful to them. Secondly I would also like to thank my parents and friends who helped me a lot in finalizing this project within the limited time frame. im of the Project To study the Drug resistance in bacteria using Antibiotics. Introduction What is Antibiotic? An antibiotic is an agent that either kills or inhibits the growth of a microorganism. The term antibiotic was first used in 1942 by Selman Waksman and his collaborators in journal articles to describe any substance produced by a microorganism that is antagonistic to the growth of other microorganisms in high dilution.[3] This definition excluded substances that kill bacteria but that are not produced by microorganisms (such as gastric juices and hydrogen peroxide). It also excluded synthetic antibacterial compounds such as the sulfonamides. Many antibacterial compounds are relatively small molecules with a molecular weight of less than 2000 atomic mass units. With advances in medicinal chemistry, most modern antibacterial are semi synthetic modifications of various natural compounds.[4] These include, for example, the beta- lactam antibiotics, which include the penicillin (produced by fungi in the genus Penicillium ), the cephalosporin, and the carbapenems. Compounds that are still isolated from living organisms are the amino glycosides, whereas other antibacterial —for example, the sulfonamides, the quinolones, and the oxazolidinones —are produced solely by chemical synthesis. In accordance with this, many antibacterial compounds are classified on the basis of chemical/biosynthetic origin into natural, semi synthetic, and synthetic. Another classification system is based on biological activity; in this classification, antibacterial are divided into two broad groups according to their biological effect on microorganisms: Bactericidal agents kill bacteria, and bacteriostatic agents slow down or stall bacterial growth. What is Antibiotic Resistance? Antibiotic resistance is a form of drug resistance whereby some (or, less commonly, all) sub-populations of a microorganism, usually a bacterial species, are able to survive after exposure to one or more antibiotics; pathogens resistant to multiple antibiotics are considered multidrug resistant (MDR) or, more colloquially, superbugs. Antibiotic resistance is a serious and growing phenomenon in contemporary medicine and has emerged as one of the pre-eminent public health concerns of the 21st century, in particular as it pertains to pathogenic organisms (the term is especially relevant to organisms that cause disease in humans). A World Health Organization report released April 30, 2014 states, "this serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country. Antibiotic resistance –when bacteria change so antibiotics no longer work in people who need them to treat infections –is now a major threat to public health." In the simplest cases, drug-resistant organisms may have acquired resistance to first-line antibiotics, thereby necessitating the use of second-line agents. Typically, a first-line agent is selected on the basis of several factors including safety, availability, and cost; a second-line agent is usually broader in spectrum, has a less favorable riskbenefit profile, and is more expensive or, in dire circumstances, may be locally unavailable. In the case of some MDR pathogens, resistance to second- and even thirdline antibiotics is, thus, sequentially acquired, a case quintessentially illustrated byStaphylococcus aureus in some nosocomial settings. Some pathogens, such as Pseudomonas aeruginosa , also possess a high level of intrinsic resistance. It may take the form of a spontaneous or induced genetic mutation, or the acquisition of resistance genes from other bacterial species by horizontal gene transfer via conjugation, transduction, or transformation. Many antibiotic resistance genes reside on transmissible plasmids, facilitating their transfer. Exposure to an antibiotic naturally selects for the survival of the organisms with the genes for resistance. In this way, a gene for antibiotic resistance may readily spread through an ecosystem of bacteria. Antibiotic-resistance plasmids frequently contain genes conferring resistance to several different antibiotics. This is not the case for Mycobacterium tuberculosis , the bacteria that causes Tuberculosis, since evidence is lacking for whether these bacteria have plasmids. Also M. tuberculosis lack the opportunity to interact with other bacteria in order to share plasmids. Genes for resistance to antibiotics, like the antibiotics themselves, are ancient. However, the increasing prevalence of antibiotic-resistant bacterial infections seen in clinical practice stems from antibiotic use both within human medicine and veterinary medicine. Any use of antibiotics can increase selective pressure in a population of bacteria to allow the resistant bacteria to thrive and the susceptible bacteria to die off. As resistance towards antibiotics becomes more common, a greater need for alternative treatments arises. However, despite a push for new antibiotic therapies, there has been a continued decline in the number of newly approved drugs. Antibiotic resistance therefore poses a significant problem. The growing prevalence and incidence of infections due to MDR pathogens is epitomized by the increasing number of familiar acronyms used to describe the causative agent and sometimes the infection; of these, MRSA is probably the most well-known, but others including VISA (vancomycinintermediate S. aureus), VRSA (vancomycin-resistant S. aureus), ESBL (Extended spectrum beta-lactamase), VRE (Vancomycin-resistant Enterococcus ) and MRAB (Multidrugresistant A. baumannii) are prominent examples. Nosocomial infections overwhelmingly dominate cases where MDR pathogens are implicated, but multidrugresistant infections are also becoming increasingly common in the community. Although there were low levels of preexisting antibioticresistant bacteria before the widespread use of antibiotics,[7] [8] evolutionary pressure from their use has played a role in the development of multidrug-resistant varieties and the spread of resistance between bacterial species.[9] In medicine, the major problem of the emergence of resistant bacteria is due to misuse and overuse of antibiotics.[10] In some countries, antibiotics are sold over the counter without a prescription, which also leads to the creation of resistant strains. Other practices contributing to resistance include antibiotic use in livestock feed to promote faster growth.[11][12] Household use of antibacterial in soaps and other products, although not clearly contributing to resistance, is also discouraged (as not being effective at infection control).[13] Unsound practices in the pharmaceutical manufacturing industry can also contribute towards the likelihood of creating antibiotic-resistant strains.[14] The procedures and clinical practice during the period of drug treatment are frequently flawed — usually no steps are taken to isolate the patient to prevent re-infection or infection by a new pathogen, negating the goal of complete destruction by the end of the course[15] (see Healthcare-associated infections and Infection control). Certain antibiotic classes are highly associated with colonization with "superbugs" compared to other antibiotic classes. A superbug, also called multiresistant, is a bacterium that carries several resistance genes .[16] The risk for colonization increases if there is a lack of susceptibility (resistance) of the superbugs to the antibiotic used and high tissue penetration, as well as broad-spectrum activity against "good bacteria". In the case of MRSA, increased rates of MRSA infections are seen with glycopeptides, cephalosporins, and especially quinolones.[17][18] In the case of colonization with Clostridium difficile , the high-risk antibiotics include cephalosporins and in particular quinolones and clindamycin.[19][20] Of antibiotics used in the United States in 1997, half were used in humans and half in animals; in 2013, 80% were used in animals. Need of this Experiment Antibiotic resistance is becoming more and more common. Antibiotics and antimicrobial agents are drugs or chemicals that are used to kill or hinder the growth of bacteria, viruses, and other microbes. Due to the prevalent use of antibiotics, resistant strains of bacteria are becoming much more difficult to treat. These "super bugs" represent a threat to public health since they are resistant to most commonly used antibiotics. Current antibiotics work by disrupting so-called cell viability processes. Disruption of cell membrane assembly or DNA translation are common modes of operation for current generation antibiotics. Bacteria are adapting to these antibiotics making them ineffective means for treating these types of infection. For example, Staphylococcus aureus have developed a single DNA mutation that alters the organism's cell wall. This gives them the ability to withstand antibiotic cell disruption processes. Antibiotic resistant Streptococcus pneumoniae produce a protein called MurM, which counteracts the effects of antibiotics by helping to rebuild the bacterial cell wall. Fighting Antibiotic Resistance Researchers are attempting to develop new types of antibiotics that will be effective against resistant strains. These new antibiotics would target the bacteria's ability to become virulent and infect the host cell. Researchers at Brandeis University have discovered that bacteria have protein "switches" that when activated, turn "ordinary" bacteria into pathogenic organisms. These switches are unique in bacteria and are not present in humans. Since the switch is a short-lived protein, elucidating its structure and function was particularly difficult. Using nuclear magnetic resonance (NMR) spectroscopy, the researchers were able to regenerate the protein for one and one half days. By extending the time frame that the protein was in its "active state," the researchers were able to map out its structure. The discovery of these "switches" has provided a new target for the development of antibiotics which focus on disrupting the activation of the protein switches. Monash University researchers have demonstrated that bacteria contain a protein complex called Translocation and Assembly Module (TAM). TAM is responsible for exporting disease causing molecules from the inside of the bacterial cell to the outer cell membrane surface. TAM has been discovered in several antibiotic resistant bacteria. The development of new drugs to target the protein would inhibit infection without killing the bacteria. The researchers contend that keeping the bacteria alive, but harmless, would prevent the development of antibiotic resistance to the new drugs. Researchers from the NYU School of Medicine are seeking to combat antibiotic resistance by making resistant bacteria more vulnerable to current antibiotics. They discovered that bacteria produce hydrogen sulfide as a means to counter the effects of antibiotics. Antibiotics cause bacteria to undergo oxidative stress, which has toxic effects on the microbes. The study revealed that bacteria produce hydrogen sulfide as a way to protect themselves against oxidative stress and antibiotics. The development of new drugs to target bacterial gas defenses could lead to the reversal of antibiotic resistance in pathogens such asStaphylococcus and E.coli. These studies indicate how highly adaptable bacteria are in relation to the application of antimicrobial treatments. Antibiotic-resistant bacteria have become a problem not only in hospitals, but in the food industry as well. Drugresistant microbes in medical facilities lead to patient infections that are more costly and difficult to treat. Resistant bacteria in turkey and other meat products have caused serious public health safety issues. Some bacteria may develop resistance to a single antibiotic agent or even multiple antibiotic agents. Some have even become so resistant that they are immune to all current antibiotics. Understanding how bacteria gain this resistance is key to the development of improved methods for treating antibiotic resistance. Material Required for the experiment 1. Sterilized Petri dishes 2. Sterilized culture tubes with media 3. Transfer loops 4. Forceps 5. Flask 6. Beaker 7. Burner 8. Penicillin 9. Aureomycin 10. Hay 11. Alcohol 12. Agar 13. Starch 14. Distilled water EXPERIMENTAL PROCEDURE 1. To 200ml of distilled water in a flask, I added 8 grams of agar powder and 2 grams of starch. Then putting a few pieces of dry hay into the medium I covered the flask with an Inverted beaker. Boiling the medium for 5 minutes and then cooling the medium to room temperature. After that placing the flask in a warm place. Within 2-3 days, formation of scum of cloudy suspension appeared on the medium indicating the growth of Bacillus subtilis. 2. Taking culture tubes with agar medium and heating the test tubes in warm water to melt agar. Cooling each test tube so that I can hold it in my hand and the agar remains liquid. After that removing the cotton plug and I passed the mouth of the test tube through the burner flame twice. Flaming the transfer loop after dipping it in alcohol and I let it cooled. After that picking up a loop full of bacterial culture from flask and then I transferred it to the warm agar in the culture tube. Flaming the loop and the mouth of the culture tube and then I replaced the cotton plug. Rolling the culture tube of warm agar between palms to I mixed the bacteria well with agar. *Transferring the bacteria should be done as quickly as possible. 3. After that I took sterilized petridishes. Removing the cotton plug and flamed the mouth of the culture tube. Then I lifted the cover of the Petridish at an angle 45 Degree and then quickly pouring the medium of the culture tube into the bottom half the dish. Removing the culture tube and replacing the cover tube into the bottom half of the dish. Removing the culture tube, and replace the cover of the Petridish. Moving the covered Petridish along the table top to distribute the medium evenly. Then I allowed the agar to cool. After that I prepared two petridishes and marked them A & B. 4. I prepared Penicillin and Aureomycin solution by dissolving the powdered drugs in distilled water. Then I cut down a few discs of filter paper of 1 cm diameter. Then I soaked a disc in each of the penicillin and Aureomycin solutions. Dipping the forceps in alcohol and the I pass ed the forceps’ tip quickly over the burner flame. Using the sterilized forceps I put Penicillin and Aureomycin soaked discs at two distant sites of Petridish A. Considering Petridish B as control. Then I kept both the Petridishes undistributed in warm place to allow the bacteria to grow. Then I observed the Petridishes for several days. OBSERVATION: The area around the antibiotic discs in the Petridishes will be clear. In other areas, colonies of bacteria will be observed. Then I examined the clear area in each Petridishes for few more days. A few very colonies may appear in the clear areas. These are the colonies of resistant strains of the bacteria. CONCLUSION: Antibiotic drugs killed most of the bacterial strain, hence the areas appeared clear. However, a few strains which were resistant in the bacterial population survived and produced colonies later. This proves the resistant strain to antibiotics were present in the bacterial population. REFERENCES: 1. Comprehensive Laboratory Manual In Biology-XII 2. Biology Text For Class XII – NCERT SITES USED 1. http://www.wikipedia.org/ 2. http://www.sciencedaily.com/articles/a/antibiotic_resistance.htm 3. http://www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Anti biotic_resistant_bacteria 4. http://www.rxlist.com/antibiotic_resistance-page3/drugscondition.htm