Lysine Production: Strain Improvement via Metabolic Engineering
advertisement

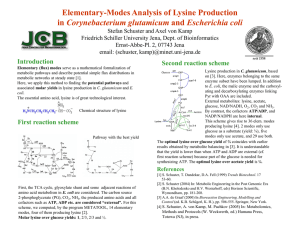
Strain improvement by metabolic engineering: lysine production as a case study for systems biology Mattheos Koffas1 and Gregory Stephanopoulos2 A central goal of systems biology is the elucidation of cell function and physiology through the integrated use of broad based genomic and physiological data. Such systemic approaches have been employed extensively in the past, as they are a central element of metabolic flux analysis, the distribution of kinetic control in pathways, and the key differentiating characteristic of metabolic engineering. In one case study, these tools have been applied to the improvement of lysine-producing strains of Corynebacterium glutamicum. The systematic study of the physiology of this organism allowed the identification of specific metabolic targets and subsequently led to significant improvements in product yield and productivity. This case study can serve as a guide for the development of systems biology tools for the utilization of large volumes of cell- and genome-wide transcriptional and physiological data. Addresses 1 Department of Chemical and Biological Engineering, University at Buffalo, The State University of New York, 904 Furnas Hall, Buffalo, New York 14260, USA 2 Department of Chemical Engineering, Massachusetts Institute of Technology, Room 56-469, Cambridge, Massachussetts 02139, USA Corresponding author: Koffas, Mattheos (mkoffas@eng.buffalo.edu) Current Opinion in Biotechnology 2005, 16:361–366 This review comes from a themed issue on Systems biology Edited by Hans V Westerhoff Available online 16th May 2005 0958-1669/$ – see front matter # 2005 Elsevier Ltd. All rights reserved. DOI 10.1016/j.copbio.2005.04.010 Introduction L-Lysine is an essential amino acid that has to be available in sufficient amounts in feed-stuffs to meet the nutritional requirements of animals and humans. This supplementation is realized by the direct addition of lysine and, as a result, a tremendous growth in the market has taken place in the past ten years. It is estimated that more than 600 000 metric tons of lysine are produced annually and, owing to the exploitation of new uses in pharmaceuticals, cosmetics and polymer materials, the market shows a growth potential of 7–10% per year. In 1956, a remarkable soil bacterium, Corynebacterium glutamicum, capable of producing large amounts of gluwww.sciencedirect.com tamic acid was isolated by a researcher at Kyowa Hakko Kogyo Co. in Japan. As both glutamic acid and lysine are derived from tricarboxylic acid (TCA) cycle metabolites, such a glutamic acid overproducing strain soon allowed the development of C. glutamicum lysine overproducers. Since then, a growing number of companies and academic researchers have carried out research aimed at the development of more efficient L-lysine production platforms. In this review we shall summarize some of the major accomplishments involving the use of systems biology approaches to optimize L-lysine biosynthesis with an emphasis on research carried out since 2002. Early studies on Corynebacterium glutamicum The soil bacterium that is currently used for the biosynthesis of L-lysine was initially classified as Micrococcus glutamicus, but is known today as Corynebacterium glutamicum [1]. As early as 1958, mutant auxotrophs (such as homoserine auxotrophs) and later regulatory mutants of this strain were developed that are capable of high rates of amino acid production. Since then, C. glutamicum mutants have become the sole producers of L-lysine manufactured today. The initial auxotrophs were developed through empirical, iterative procedures involving mutagenesis and selection [2]. Strain optimization was performed by random approaches (chemical mutagenesis or UV irradiation) to release key enzymes from strict metabolite regulation (feedback inhibition) [3]. The resulting strains were grown in small-scale fermentations to select optimal producers and this recursive procedure would be repeated for the newly optimized strains. Strains developed by such approaches could reach relatively low conversion yields of approximately 0.2–0.3 g of L-lysine (g sugar) 1 [4,5]. Later on, the disadvantage of supplementing the defined medium with large amounts of the auxotrophic amino acids would be overcome by developing ‘leaky strains’. Such strains would synthesize a limited amount of the required metabolite and, as a result, its intracellular level would be low, thus avoiding feedback inhibition and repression of key enzymes in the lysine biosynthetic pathway. Finally, cell fusion using the method of protoplast fusion has also been applied for the successful development of industrial lysine producers [6]. Metabolite balancing Though successful, random mutational approaches were uncertain and tedious. A more rational design of lysine overproducers was initiated in the 1980s and was based on biochemical and physiological measurements, usually Current Opinion in Biotechnology 2005, 16:361–366 362 Systems biology obtained from continuous culture experiments. Using these data, a mathematical formulation based on mass balances of extracellular substrate consumption and product formation rates was developed to analyze the complex metabolic network of lysine biosynthesis [7]. This approach offered a more rational methodology for strain design and allowed the consolidation and validation of metabolic networks, the identification of key branch points, and the determination of L-lysine maximum theoretical yields. In the first such attempt to analyze the metabolic network, stoichiometrically based mass balances were applied by Vallino and Stephanopoulos in C. glutamicum fermentation experiments to determine metabolic fluxes and to identify potential metabolic bottlenecks [8,9]. Some of the main contributions of this work were the solidification of the C. glutamicum biochemistry (guided mainly by enzyme assays), the determination of basal metabolic flux distributions during growth and lysine biosynthesis, the characterization of the principal nodes of the lysine biosynthetic pathway, and the determination of the maximum possible lysine yield [8–12]. Carbon flux distributions resulting from metabolic perturbations demonstrated that lysine yield appeared to be constrained by the flexibility of the phosphoenol pyruvate (PEP)/pyruvate node. Moreover, it was shown that the rate of lysine biosynthesis was always less than or equal to the rate of oxaloacetate synthesis via the anaplerotic pathways. The value of the intracellular (or metabolic) flux analysis that was initiated by this research is evident in the diversity of applications that have since made use of this approach. The main weakness of the extracellular metabolite balancing method was its inability to provide flux estimates in cases of metabolic network structural singularities (i.e. where the metabolic network is structured such that simple metabolite balances cannot provide flux estimation for separate reactions that lead to the same product from different substrates). For example, it was not possible to estimate carbon fluxes through the PEP carboxylase and pyruvate carboxylase anaplerotic reactions. In addition, the rates of extracellular metabolite excretion and consumption could only provide net fluxes, while no information about the extent of reversibility of a reaction could be obtained. Finally, assumptions about the cellular energy balance had to be included, thus raising further uncertainties. To overcome these problems, additional experimental data were required to establish a reliable stationary flux analysis: isotopic tracer experiments have emerged as a prominent tool for this purpose. Isotopic tracer methods The advantage of introducing stable isotopic labeling methods is that the label can be traced from substrate Current Opinion in Biotechnology 2005, 16:361–366 to product with a specific pattern that is completely dependent upon the structure and fluxes of the biochemical pathway reactions. Thus, labeling methods can be used to determine flux distribution in structurally ‘singular’ groups as well as to elucidate the reversibility of intracellular reactions. These more refined methods can also validate the flux estimates and accompanying assumptions used in the overall material balance technique. In one of the first efforts to use isotopomer labeling to elucidate the C. glutamicum physiology, the nuclear magnetic resonance (NMR) technique was applied by Yamaguchi et al. [13] to study actual flux distributions in two routes of lysine biosynthesis: the one-step diaminopimelate dehydrogenase route and the four-step succinylase route (Figure 1). The authors found that the one-step route accounted for 30–40% of the flux towards lysine. Later, by using knockout mutants and recombinant strains, Shaw-Reid et al. [14] established that the fourstep route is dispensable for lysine production. In a similar analysis, Sonntag et al. [15] fed cultures with (6-13C)glucose and showed that flux contribution to the one-step route (diaminopimelate dehydrogenase) decreased from 72% to zero over the course of shake flask culture time. This was traced to the availability of ammonium in the culture medium. Together, these studies demonstrate the powerful use of the NMR technique to determine accurate split ratios of pathways. Moving to the more challenging problem of analysis of the central carbon metabolism of C. glutamicum, Park and colleagues [16] developed and used a mathematical model to study isotopomer distributions of TCA cycle intermediates following the administration of 13C-labeled substrates. Previous studies had utilized isotopomer labeling to quantify fluxes at the PEP/pyruvate node and to quantify exchange fluxes. However, these studies either investigated cases in which only a single flux was considered in each direction, or cases in which parallel reactions were lumped together. Applying this approach to select genetic backgrounds, Park and colleagues were able not only to identify a novel pyruvate carboxylating anaplerotic pathway in C. glutamicum, but also demonstrated that 90% of total anaplerotic activity resulted from pyruvate carboxylation. This result was later confirmed in wild-type C. glutamicum lysine-producing strains [17] by utilizing a thorough and universal model for carbon isotopomer labeling experiments, which takes into account the bidirectionality of biochemical reactions [18–20]. The modeling work complemented earlier physiological data suggesting that the only other anaplerotic reaction in C. glutamicum, catalyzed by phosphoenolpyruvate carboxylase, is dispensable for lysine biosynthesis [21]. Separate studies have confirmed the presence of pyruvate carboxylase in C. glutamicum [22,23] and its overexpression confirmed that this anaplerotic reaction plays a key role in lysine biosynthesis [24,25]. www.sciencedirect.com Strain improvement by metabolic engineering Koffas and Stephanopoulos 363 Figure 1 O OH HO Aspartate O NH2 ATP Aspartate kinase ADP O O OH O L-4-Aspartylphosphate NH2 HO P OH O NADPH Aspartate semialdehyde dehydrogenase NADP O H HO L-Aspartate-4-semialdehyde NH2 Dihydrodipicolinate synthase O Pyruvate L-2,3,-Dihydrodipicolinate HO N COOH O NADPH Dihydrodipicolinate reductase NADP L-2,3,4,5-Tetrahydrodipicolinate HO N COOH Succinyl-CoA O CoA Tetrahydrodipicolinate succinylase O O HN HO COOH N-Succinyl-2-L-amino-6oxopimelate COOH O NH4+ Glutamate Succinyl-amino ketopimelate transaminase NADPH Oxoglutarate O NADP N-Succinyl-LL-2,6diaminopimelate COOH HO Diaminopimelate dehydrogenase COOH HN NH2 O Succinyl-amino pimelate succinylase Succinate O COOH HO NH2 LL-2,6-Diaminopimelate H2N Diaminopimelate epimerase O COOH HO NH2 D,L-2,6-Diaminopimelate H2N Diaminopimelate decarboxylase CO2 O NH2 HO L-Lysine NH2 Current Opinion in Biotechnology The L-lysine biosynthetic pathway in Corynebacterium glutamicum. www.sciencedirect.com Current Opinion in Biotechnology 2005, 16:361–366 364 Systems biology Despite the success stories, NMR-based approaches are limited by their low sensitivity: labeling patterns are mostly analyzed from amino acids obtained from hydrolyzed cellular proteins [26]. In this respect, labeling patterns of intermediary metabolites, usually occurring at low concentrations, are difficult to measure directly [27]. To address this limitation, mass spectrometry (MS) methods have emerged as a powerful alternative for analyzing labeling patterns: these methods require small amounts of sample and are faster and are more sensitive than NMR [28]. These features make MS suitable for the measurement of intracellular metabolite labeling patterns, and thus for the investigation of dynamic responses of the metabolism to defined changes of cultivation conditions. The application of MS for identifying the topology of metabolic networks has been successfully demonstrated in many different organisms, including C. glutamicum during lysine biosynthesis. Wittmann and Heinzle [29] first determined metabolite fluxes in the lysine biosynthetic network using MALDI-TOF (matrix-assisted laser desorption ionization time-of-flight) MS, although they were unable to resolve all the metabolic network singularities. Later on, gas chromatography MS was introduced to perform a comparative metabolic network analysis of a genealogy of five successive generations of lysine-producing C. glutamicum strains [30] and to make comparative studies between growth on glucose and fructose [31]. It appears, however, that the major potential of MS analysis lies in its applicability to high-throughput metabolic flux analysis that will allow broad quantitative screening of lysine-producing C. glutamicum mutants [32]. Genome sequencing and functional genomics The completion of the genome sequence of C. glutamicum provides a leap forward both for understanding the biology of the organism and for enabling further metabolic engineering for the production of lysine and other biochemical products [33,34,35]. Annotation of the genome sequence provided valuable hints for missing metabolic steps in the lysine biosynthetic pathway, while comparative genomics allowed the identification of beneficial mutations for the improvement of lysine production [36,37]. In an excellent example of inverse metabolic engineering, a comparative genomics analysis between an engineered lysine-producing strain and its parental strain identified beneficial point mutations that were then used to guide the construction of a high lysine-producing strain [38,39]. When the fermentation temperature was raised to 40 8C, lysine production increased even further and reached 85 g/L within 28 h [40]. The availability of the whole genome sequence provided the necessary tools for performing genome-wide expression analyses (functional genomics). The use of DNA microarrays in the elucidation of C. glutamicum transcripCurrent Opinion in Biotechnology 2005, 16:361–366 tion regulation has been reviewed extensively by Wendisch and colleagues and has already been utilized in efforts to develop valine-producing strains [41,42]. Furthermore, global transcription profiling has been employed for elucidating C. glutamicum physiology; for example, 92 genes that are up- or downregulated during phosphate starvation were identified [43] and the effect of the deletion of acnR, a TetR-type repressor of the aconitase gene, was assessed [44]. Other related studies have revealed the effect of deletions of signal transduction systems [45] and the effect of deletion of the pyruvate kinase ( pyk) gene [46] on the C. glutamicum transcriptome. The expanded view of C. glutamicum physiology, made possible by genome sequencing and parallel, highthroughput technologies, has facilitated the application of a more holistic approach. Such an approach will allow links between the different components of cell physiology, such as the transcriptome and fluxome, to be elucidated [47]. It is expected that such a systems approach will prove a very useful adjunct in designing optimized C. glutamicum strains for maximal lysine production, in a similar way to that already demonstrated for other important industrial strains and applications [48]. Conclusions It might be noted that the topic of this issue, systems biology, was hardly mentioned in this article. Yet, a systemic approach to the analysis of C. glutamicum physiology and the improvement of lysine-producing strains is a recurrent theme of the presented work that spans approximately two decades [49]. The analysis and determination of fluxes through an integrated reconstruction of the biosynthetic and central carbon metabolic pathways of the organism was one of the first demonstrations of a systems approach to the analysis of microbial physiology. Furthermore, the calculation of theoretical maximum yields and the identification of key branch points harboring control of the entire network flux could not have been accomplished without a systems mindframe. Finally, perhaps the most outstanding illustration of the application of systems concepts that led to the improvement of lysine-production strains is the coordinated overexpression of two genes (encoding pyruvate carboxylase and aspartokinase), in realization of the fact that pathway flux control is shared and not localized to any particular enzyme [25]. This resulted in a marked improvement in specific lysine productivity, where many similar efforts had failed in the past. We offer this case study as an example of what can be accomplished by systems-oriented approaches to strain improvement. The need for such approaches will be intensified in the near future in light of the large volumes of data that will be generated from cell-wide and genomewide measurements. On the one hand, we do not yet have good methods that will allow us to make judicious use of www.sciencedirect.com Strain improvement by metabolic engineering Koffas and Stephanopoulos 365 this avalanche of information whereas, on the other hand, there is a sufficient arsenal of tools that can be applied to smaller scale, more focused investigations. We believe that significant progress can be accomplished in strain improvement with these methods, while more powerful approaches are being developed that will allow a more complete view of the metabolic machinery of microorganisms and will facilitate their modification for industrial applications by metabolic engineering. 15. Sonntag K, Eggeling L, De Graaf AA, Sahm H: Flux partitioning in the split pathway of lysine synthesis in Corynebacterium glutamicum. Quantification by 13C- and 1H-NMR spectroscopy. Eur J Biochem 1993, 213:1325-1331. References and recommended reading 18. Wiechert W, De Graaf AA: Bidirectional reaction steps in metabolic networks: I. Modeling and simulation of carbon isotope labeling experiments. Biotechnol Bioeng 1997, 55:101-117. Papers of particular interest, published within the annual period of review, have been highlighted as: of special interest of outstanding interest 16. Park SM, Shaw-Reid CA, Sinskey AJ, Stephanopoulos G: Elucidation of anaplerotic pathways in Corynebacterium glutamicum via 13C-NMR spectroscopy and GC-MS. Appl Microbiol Biotechnol 1997, 47:430-440. 17. Petersen S, de Graaf AA, Eggeling L, Mollney M, Wiechert W, Sahm H: In vivo quantification of parallel and bidirectional fluxes in the anaplerosis of Corynebacterium glutamicum. J Biol Chem 2000, 275:35932-35941. 19. Wiechert W, De Graaf AA: Bidirectional reaction steps in metabolic networks: II. Flux estimation and statistical analysis. Biotechnol Bioeng 1997, 55:118-135. 1. Lessard PA, Guillouet S, Willis LB, Sinskey AJ: Corynebacteria, Brevibacteria. In Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis and Bioseparation, Vol 1. Edited by Michael C, Flickinger SWD. Wiley; 1999:729-740. 20. Wiechert W, Mollney M, Isermann N, Wurzel M, de Graaf AA: Bidirectional reaction steps in metabolic networks: III. Explicit solution and analysis of isotopomer labeling systems. Biotechnol Bioeng 1999, 66:69-85. 2. Nakayama K: Lysine. In Comprehensive Biotechnology, Vol.3. Edited by Blanch HW, Drew S, Wang DIC. Pergamon Press; 1985: 607-620. 3. Shiio I, Yoshino H, Sugimoto S: Isolation and properties of lysine-producing mutants with feedback-resistant aspartokinase derived from a Brevibacterium flavum strain with citrate synthase and pyruvate kinase defects and feedback resistant phosphoenol pyruvate carboxylase. Agric Biol Chem 1990, 54:3275-3282. 21. Gubler M, Park SM, Jetten M, Stephanopoulos G, Sinskey AJ: Effects of phosphoenol pyruvate carboxylase deficiency on metabolism and lysine production in Corynebacterium glutamicum. Appl Microbiol Biotechnol 1994, 40:857-863. 4. Pfefferle W, Mockel B, Bathe B, Marx A: Biotechnological manufacture of lysine. Adv Biochem Eng Biotechnol 2003, 79:59-112. 5. Kiss RD, Stephanopoulos G: Metabolic characterization of a L-lysine producing strain by continuous culture. Biotechnol Bioeng 1992, 39:565-574. 6. Karasawa M, Tosaka O, Ikeda S, Yoshi H: Application of protoplast fusion to the development of L-threonine and L-lysine producers. Agric Biol Chem 1986, 50:339-346. 7. Stephanopoulos G: Metabolic fluxes and metabolic engineering. Metab Eng 1999, 1:1-11. 8. Vallino JJ, Stephanopoulos G: Flux determination in cellular bioreaction networks: applications to lysine fermentations. In Frontiers in Bioprocessing, Vol.1. Edited by Sikdar S, Bier M, Todd P. CRC Press; 1989:205-219. 9. Vallino JJ, Stephanopoulos G: Metabolic flux distributions in Corynebacterium glutamicum during growth and lysine overproduction. Reprinted from Biotechnology and Bioengineering, Vol. 41, 633-646 (1993). Biotechnol Bioeng 2000, 67:872-885. 10. Vallino JJ, Stephanopoulos G: Carbon flux distributions at the pyruvate branch point in Corynebacterium glutamicum during lysine overproduction. Biotechnol Prog 1994, 10:320-326. 11. Vallino JJ, Stephanopoulos G: Carbon flux distributions at the glucose 6-phosphate branch point in Corynebacterium glutamicum during lysine overproduction. Biotechnol Prog 1994, 10:327-334. 12. Stephanopoulos G, Vallino JJ: Network rigidity and metabolic engineering in metabolite overproduction. Science 1991, 252:1675-1681. 22. Peters-Wendisch PG, Wendisch VF, Paul S, Eikmanns BJ, Sahm H: Pyruvate carboxylase as an anaplerotic enzyme in Corynebacterium glutamicum. Microbiology 1997, 143:1095-1103. 23. Koffas MA, Ramamoorthi R, Pine WA, Sinskey AJ, Stephanopoulos G: Sequence of the Corynebacterium glutamicum pyruvate carboxylase gene. Appl Microbiol Biotechnol 1998, 50:346-352. 24. Koffas MA, Jung GY, Aon JC, Stephanopoulos G: Effect of pyruvate carboxylase overexpression on the physiology of Corynebacterium glutamicum. Appl Environ Microbiol 2002, 68:5422-5428. 25. Koffas MA, Jung GY, Stephanopoulos G: Engineering metabolism and product formation in Corynebacterium glutamicum by coordinated gene overexpression. Metab Eng 2003, 5:32-41. The coordinated overexpression of aspartate kinase and pyruvate carboxylase in this work led to the most significant increase in lysine biosynthesis in C. glutamicum to date. 26. Szyperski T: Biosynthetically directed fractional 13C-labeling of proteinogenic amino acids. An efficient analytical tool to investigate intermediary metabolism. Eur J Biochem 1995, 232:433-448. 27. Follstad BD, Stephanopoulos G: Effect of reversible reactions on isotope label redistribution – analysis of the pentose phosphate pathway. Eur J Biochem 1998, 252:360-371. 28. Klapa MI, Aon JC, Stephanopoulos G: Systematic quantification of complex metabolic flux networks using stable isotopes and mass spectrometry. Eur J Biochem 2003, 270:3525-3542. 29. Wittmann C, Heinzle E: Application of MALDI-TOF MS to lysine-producing Corynebacterium glutamicum: a novel approach for metabolic flux analysis. Eur J Biochem 2001, 268:2441-2455. 13. Yamaguchi K, Ishino S, Araki K, Shirahata K: 13C NMR studies of lysine fermentation with a Corynebacterium glutamicum mutant. Agric Biol Chem 1986, 50:2453-2459. 30. Wittmann C, Heinzle E: Genealogy profiling through strain improvement by using metabolic network analysis: metabolic flux genealogy of several generations of lysine-producing Corynebacteria. Appl Environ Microbiol 2002, 68:5843-5859. 14. Shaw-Reid CA, McCormick MM, Sinskey AJ, Stephanopoulos G: Flux through the tetrahydrodipicolinate succinylase pathway is dispensable for L-lysine production in Corynebacterium glutamicum. Appl Microbiol Biotechnol 1999, 51:325-333. 31. Kiefer P, Heinzle E, Zelder O, Wittmann C: Comparative metabolic flux analysis of lysine-producing Corynebacterium glutamicum cultured on glucose or fructose. Appl Environ Microbiol 2004, 70:229-239. www.sciencedirect.com Current Opinion in Biotechnology 2005, 16:361–366 366 Systems biology 32. Wittmann C, Kim HM, Heinzle E: Metabolic network analysis of lysine producing Corynebacterium glutamicum at a miniaturized scale. Biotechnol Bioeng 2004, 87:1-6. 33. Ikeda M, Nakagawa S: The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 2003, 62:99-109. Two articles [33,34] describe the sequencing of the C. glutamicum genome by two separate groups in Japan and Europe. 34. Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L et al.: The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 2003, 104:5-25. See annotation for [33]. 35. Tauch A, Homann I, Mormann S, Ruberg S, Billault A, Bathe B, Brand S, Brockmann-Gretza O, Ruckert C, Schischka N et al.: Strategy to sequence the genome of Corynebacterium glutamicum ATCC 13032 use of a cosmid and a bacterial artificial chromosome library. J Biotechnol 2002, 95:25-38. 36. Nishio Y, Nakamura Y, Usuda Y, Sugimoto S, Matsui K, Kawarabayasi Y, Kikuchi H, Gojobori T, Ikeo K: Evolutionary process of amino acid biosynthesis in Corynebacterium at the whole genome level. Mol Biol Evol 2004, 21:1683-1691. 37. Hartmann M, Tauch A, Eggeling L, Bathe B, Mockel B, Puhler A, Kalinowski J: Identification and characterization of the last two unknown genes, dapC and dapF, in the succinylase branch of the L-lysine biosynthesis of Corynebacterium glutamicum. J Biotechnol 2003, 104:199-211. 38. Ohnishi J, Mitsuhashi S, Hayashi M, Ando S, Yokoi H, Ochiai K, Ikeda M: A novel methodology employing Corynebacterium glutamicum genome information to generate a new L-lysine-producing mutant. Appl Microbiol Biotechnol 2002, 58:217-223. This work is an excellent demonstration of the use of inverse metabolic engineering and comparative genomics in the identification of beneficial mutations that eventually led to a significant improvement in lysine production. 39. Ohnishi J, Katahira R, Mitsuhashi S, Kakita S, Ikeda M: A novel gnd mutation leading to increased L-lysine production in Corynebacterium glutamicum. FEMS Microbiol Lett 2005, 242:265-274. Current Opinion in Biotechnology 2005, 16:361–366 40. Ohnishi J, Hayashi M, Mitsuhashi S, Ikeda M: Efficient 40 -C fermentation of L-lysine by a new Corynebacterium glutamicum mutant developed by genome breeding. Appl Microbiol Biotechnol 2003, 62:69-75. 41. Wendisch VF: Genome-wide expression analysis in Corynebacterium glutamicum using DNA microarrays. J Biotechnol 2003, 104:273-285. 42. Lange C, Rittmann D, Wendisch VF, Bott M, Sahm H: Global expression profiling and physiological characterization of Corynebacterium glutamicum grown in the presence of L-valine. Appl Environ Microbiol 2003, 69:2521-2532. 43. Ishige T, Krause M, Bott M, Wendisch VF, Sahm H: The phosphate starvation stimulon of Corynebacterium glutamicum determined by DNA microarray analyses. J Bacteriol 2003, 185:4519-4529. 44. Krug A, Wendisch VF, Bott M: Identification of AcnR, a TetRtype repressor of the aconitase gene acn in Corynebacterium glutamicum. J Biol Chem 2005, 280:585-595. 45. Moker N, Brocker M, Schaffer S, Kramer R, Morbach S, Bott M: Deletion of the genes encoding the MtrA-MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotics susceptibility and expression of genes involved in osmoprotection. Mol Microbiol 2004, 54:420-438. 46. Netzer R, Krause M, Rittmann D, Peters-Wendisch PG, Eggeling L, Wendisch VF, Sahm H: Roles of pyruvate kinase and malic enzyme in Corynebacterium glutamicum for growth on carbon sources requiring gluconeogenesis. Arch Microbiol 2004, 182:354-363. 47. Kromer JO, Sorgenfrei O, Klopprogge K, Heinzle E, Wittmann C: In-depth profiling of lysine-producing Corynebacterium glutamicum by combined analysis of the transcriptome, metabolome, and fluxome. J Bacteriol 2004, 186:1769-1784. 48. Hermann T: Using functional genomics to improve productivity in the manufacture of industrial biochemicals. Curr Opin Biotechnol 2004, 15:444-448. 49. Stephanopoulos G, Alper H, Moxley J: Exploiting biological complexity for strain improvement through systems biology. Nat Biotechnol 2004, 22:1261-1267. A very recent and thorough review of the concept of systems biology. www.sciencedirect.com