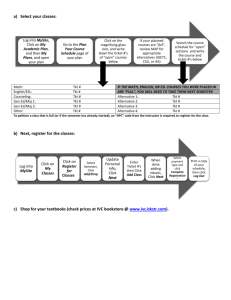
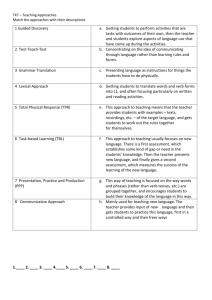
Biosynthesis of adipic acid by reversing its degradation pathway and
advertisement

Supplementary Information Metabolic Engineering of Corynebacterium glutamicum for the Production of L-ornithine Seo Yun Kim,1 Joungmin Lee,1 and Sang Yup Lee1,2 1 Metabolic and Biomolecular Engineering National Research Laboratory, Department of Chemical and Biomolecular Engineering (BK21 plus program), Center for Systems and Synthetic Biotechnology, BioProcess Engineering Research Center, and, Institute for the BioCentury, KAIST, 291 Daehakro, Yuseong-gu, Daejeon 305-701, Republic of Korea; telephone: +82-42-350-3930; fax: +82-42-350-8800; e-mail: leesy@kaist.ac.kr 2 Bioinformatics Research Center, KAIST, Daejeon, Republic of Korea Correspondence to: S.Y. Lee 1 Supplementary Materials and Methods Construction of the plasmids All primers used in this study are listed in Supplementary Table I. Pfu-X DNA polymerase (Solgent, Daejeon, Korea), restriction enzymes (New England Biolabs, Ipswich, MA), and T4 DNA ligase (Elpis Biotechnology, Daejeon, Korea) were used for the cloning. Plasmids were purified using a DNA-Spin purification kit (iNtRON Biotechnology, Daejeon, Korea), and genomic DNAs using an ExgeneTM Cell SV kit (GeneAll Biotechnology, Seoul, Korea). The DNA sequences of the constructed plasmids were confirmed by Sanger sequencing. For the construction of pSY927 and pSY223, the argCJBD genes including the native promoters were amplified with their native promoters from genomic DNA of C. glutamicum ATCC 13032 and ATCC 21831 with the primers WT_F/WT_R and AT1_F/AT1_R, respectively. Each PCR product was assembled with the pEK0 that was amplified by inverse PCR with K0_F/K0_R (Gibson et al., 2009). For the construction of pSY01, partial fragments of the proB gene were amplified from the genomic DNA of C. glutamicum ATCC 13032 with the primers ProB_LA_F/ProB_LA_R and ProB_RA_F/ProB_RA_R (Supplementary Table I). The resulting two distant homologous arms, each having a length of ca. 0.5 kb, were fused by overlapping PCR. The finally assembled fragment was cloned through Gibson assembly with the inverse PCR product of pK19mobsacB plasmid amplified with the primer pair pK19_F/R. The pSY03 plasmid (for argR knockout) was constructed in the same way as pSY01 using the primer pairs ArgR_LA_F/ ArgR_LA_R and ArgR_RA_F/ ArgR_RA_R. For the construction of the pSY02 plasmid (for argF knockout), two homologous arms of the argF gene were amplified from C. glutamicum ATCC 13032 using primers ArgF_LA_F/ ArgF_LA_R and ArgF_RA_F/ ArgF_RA_R. These two fragments are fused by overlapping 2 PCR, digested with BamHI/HindIII, and ligated into BamHI/HindIII double digested pK19mobsacB. The pSY04 and pSY05 plasmids, for the start codon replacement of the pgi and zwf genes, respectively, were constructed in the same manner as pSY01. For the construction of pSY06, two homologous regions flanking the native promoter of the tkt operon and the sod promoter were amplified from the genomic DNA of C. glutamicum ATCC 13032 with the primer pairs Tkt_LA_F/Tkt_LA_R, Tkt_RA_F/Tkt_RA_R, and Sod_F/Sod_R, respectively. These fragments were assembled together into pK19mobsacB by Gibson assembly, so that the sod promoter located between two homologous arms. Transformation and chromosomal manipulation in C. glutamicum The C. glutamicum cells was grown in 25 mL of the CR medium up to OD600 0.7 to 0.8 and harvested by centrifuge. The pellet was washed twice using 25 mL of EPB1 buffer containing 20 mM of HEPES and 5% (w/v) of glycerol. Finally, cells were resuspended in 1 mL of EPB2 buffer containing 5 mM of HEPES and 15% (w/v) of glycerol. The 0.1 ml of competent cells and DNA were mixed and put into a 2-mm gapped cuvette. The cuvette was subject to an exponential decay pulse of 1.8 kV, 200 Ω, and 25 μF. Immediately after the pulse, 1 mL of the CR medium was added into the cuvette, and the cells were cultivated at 30°C for 2 hours for the recovery. Cells were spread onto a CR agar supplemented with Km. Gene knockout or allelic replacement in C. glutamicum was conducted according to Schafer et al. (1994). A knockout plasmid or a replacement plasmid was introduced into C. glutamicum via electroporation, and single-crossover mutants were selected by kanamycin resistance. Double-crossover mutants were selected by the loss of sucrose sensitivity on CR medium supplemented with 15% (w/v) sucrose. Knockout mutants were first verified by the loss of kanamycin resistance and finally by colony PCR. Those with codon or promoter replacement were verified by sequencing of the PCR products from the mutants. 3 Supplementary Table I. Plasmids used in this study. Sequence(5’->3’) Primer pK19_F GGGCGGTTTTATGGACAGCAAGCGA pK19_R GGCGAGCGGTATCAGCTCACTCAAA AACCGTATTACCGCCTTTGAGTGAGCTGATACCGCTCGCCAACG ProB_LA_F ATGGTAACGCTATGAA ProB_LA_R ATACCGGGCAAAAGAACGTCCCC ProB_RA_F GACGTTCTTTTGCCCGGTATTTGTATGCCGCAGATACTGCAGGA GCTGGCAATTCCGGTTCGCTTGCTGTCCATAAAACCGCCCCGGC ProB_RA_R AAATCCGGGAACTCCG ArgF_LA_F ACCGAAGCTTGTTGTTCCCGATGTGGTGAC ArgF_LA_R ATCATCAGCCAGAAAATGGC CGCCATTTTCTGGCTGATGATCAGCAGGCCTTAAGGGTAAGAAG ArgF_RA_F G ArgF_RA_R TCAAGGATCCTTACCTCGGCTGGTTGGCCA AACCGTATTACCGCCTTTGAGTGAGCTGATACCGCTCGCCTTGC ArgR_LA_F CACCGCGGGCATGGAT ArgR_LA_R AATGAGAGCTTGGCGTGCAG ArgR_RA_F CTGCACGCCAAGCTCTCATTCTCAGCGGGCGCACCACTTA GCTGGCAATTCCGGTTCGCTTGCTGTCCATAAAACCGCCCCTCA ArgR_RA_R ACGAGGTGCTTGACGA GGGCGGTTTTATGGACAGCAAGCGAGCGTTGCCAACGCTCAGC Zwf_LA_F G Zwf_LA_R GTTTGTGCTCATGATGGTA 4 Zwf_RA_F CTACCATCATGAGCACAAAC GGCGAGCGGTATCAGCTCACTCAAATGATCACGCGGCGCCATGC Zwf_RA_R T Tkt_LA_F ATGAGGATCCAGAGTGTTTACTCAAGACATTTTCTAAGA Tkt_LA_R CATTCGCAGGGTAACGGCCATCTTAAGTCTGGGAGCTGTGACG Sod_F AGCTGCCAATTATTCCGGGCTTG Sod_R TTTCCGCACCGAGCATATACATCTT Tkt_RA_F GATGTATATGCTCGGTGCGGAAATTGACCACCTTGACCTGTC Tkt_RA_R GAATTCCGCCCTCAGCAGCGG K0_F TGGGGTGCCTAATGAGTGAGCTAAC K0_R CTACGGTGTGCAATCTATTCACGAT GTATCTGGGGGTGGCATCGTGAATAGATTGCACACCGTAGAATT WT_F CATGCTTTTACCCACTTGCAGTT ACGCAATTAATGTGAGTTAGCTCACTCATTAGGCACCCCATTATG WT_R CGATTGTCTCGGCAATAG GTATCTGGGGGTGGCATCGTGAATAGATTGCACACCGTAGAATT AT1_F CATGCTTTTACCCACTTGCA ACGCAATTAATGTGAGTTAGCTCACTCATTAGGCACCCCATTATG AT1_R CGATTGTCTCGGCAATAGC 5 Table II. Production of L-ornithine by C. glutamicum strains in the flask culturea,b. Strain L-ornithine (mg/L) Cell growth (OD600) Lactic acid (g/L) Acetic acid (g/L) Wild-type 35.33 ± 0.01 35.33 ± 0.09 45.61 ± 0.12 4.67 ± 0.02 YW02 37.80 ± 0.12 37.80 ± 0.14 25.61 ± 0.12 3.61 ± 0.15 YW03 230.29 ± 0.07 33.02 ± 0.16 27.86 ± 0.08 3.32 ± 0.07 YW03 (pSY927) 1970.12 ± 0.25 23.31 ± 0.09 19.23 ± 0.03 1.32 ± 0.12 YW03 (pSY223) 7190.27 ± 0.21 31.07 ± 0.05 16.76 ± 0.07 1.60 ± 0.04 YW04 242.29 ± 0.21 29.29 ± 0.09 26.86 ± 0.18 4.38 ± 0.16 YW04 (pSY223) 6549.65 ± 0.13 22.20 ± 0.02 27.39 ± 0.11 1.91 ± 0.01 YW05 261.29 ± 0.37 24.57 ± 0.11 29.86 ± 0.27 3.72 ± 0.13 YW06 417.21 ± 0.35 19.33 ± 0.17 29.51 ± 0.14 3.29 ± 0.08 YW06 (pSY223) 8549.65 ± 0.10 23.29 ± 0.08 28.12 ± 0.09 1.86 ± 0.01 a The values were obtained by triplicate experiments; (mean) ± (SD) b Glucose (initially 50 g/L) was completely consumed in every strain. 6 Supplementary Table III. Mutations identified in the argCJBD genes of the ATCC 21831 strain compared to those in ATCC 13032 straina. Gene Type Mutation (nucleotide) Mutation (amino acid) argCa Nonsynonymous Deletion (1..30)b, C32T Deletion(1..10)a, T12I argJ Nonsynonymous C553T, T849C, T864G, G331D G992A argB Nonsynonymous T144C, A160G, M64Vc C354T, T576C, A594G, C774T, G882A argD Synonymous T94C, G1152A None a According to Park et al. (2014) b This difference might be originated from the different annotation algorithms used for the ATCC 13032 and 21832 strains; the sequence actually exists in the ATCC 21831 strain. c This mutation is identical to M31V reported by Ikeda et al. (2009) and Park et al. (2014). 7 References Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343-345. Ikeda M, Mitsuhashi S, Tanaka K, Hayashi M. 2009. Reengineering of a Corynebacterium glutamicum L-arginine and L-citrulline producer. Appl Environ Microbiol 75:1635-1641. Park SH, Kim HU, Kim TY, Park JS, Kim SS, Lee SY. 2014. Metabolic engineering of Corynebacterium glutamicum for L-arginine production. Nat Commun 5:4618. Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. 8