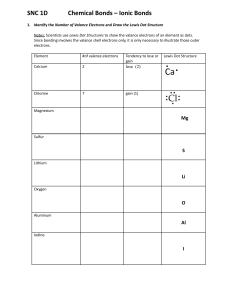
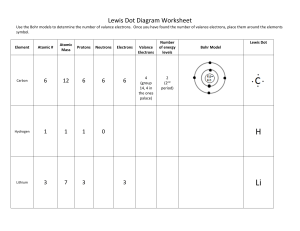
Chemical Bonds – Ionic Bonds 1. Identify the Number of Valance Electrons and Draw the Lewis Dot Structure Notes: Scientists use Lewis Dot Structures to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. Element Calcium Carbon Hydrogen Helium Oxygen Fluorine Neon Sodium Aluminum Group Number (PT) # of Valance Electrons Lewis Dot Structure Notes: Remember that Metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive “cations”. Nonmetals tend to gain electrons, filling up their current energy levels, becoming larger, forming negative “anions”. Complete the chart below. Element Na Be Cl S Al Ne K N O Ca P B Mg Lewis Dot # of Valance e- Gain/Lose ___ e- Valance Charge 1 L1 +1 Chemical Bonds – Ionic Bonds KEY 2. Identify the Number of Valance Electrons and Draw the Lewis Dot Structure Notes: Scientists use Lewis Dot Structures to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. Element Calcium Carbon Group Number (PT) IIA 2 IVA 14 Hydrogen IA 1 Helium VIIIA 18 # of Valance Electrons 2 4 2 ●● He 6 Fluorine VIIA 17 7 Sodium IA 1 Aluminum IIIA 13 ● ●C● ● 1 VIA 16 VIIIA 18 ● Ca● ● H Oxygen Neon Lewis Dot Structure ●● ●O● ●● ●● :F: ● ●● 8 :Ne: 1 ●● ● Na 3 ● ●Al● Notes: Remember that Metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive “cations”. Nonmetals tend to gain electrons, filling up their current energy levels, becoming larger, forming negative “anions”. Complete the chart below. Element Lewis Dot # of Valance e- Gain/Lose ___ e- Valance Charge Na ● Na 1 L1 +1 Be ● Be● 2 L2 +2 7 G1 -1 ●● Cl :Cl: ● ● S : S: 6 G2 -2 Al ● ● Al● ● ●● 3 L3 +3 Ne :Ne: 8 0 0 ●● ● K 1 L1 +1 5 G3 -3 :O: 6 G2 -2 ● ● Ca● 2 L2 +2 K N O Ca ●● ●N● ● ● P B Mg ●● ●P● ● ● ●B ● ● Mg ● 5 G3 -3 3 L3 +3 2 L2 +2