Chapter 3:
Fundamentals of Crystallography
1
Order: Short vs Long Range
2
Anisotropy
• The physical properties of single crystals of
some substances depend on the
crystallographic direction in which
measurements are taken.
• For example, the elastic modulus, electrical
conductivity, and the index of refraction may
have different values in the [100] and [111]
directions.
• The directionality of the properties is termed
anisotropy and is associated with the atomic
spacing.
3
Isotropic
• If measured properties are independent of
the direction of measurement then they are
isotropic.
• For many polycrystalline materials, the
crystallographic orientations of the individual
grains are totally random.
• So, though, a specific grain may be
anisotropic, when the specimen is composed
of many grains, the aggregate behavior may
be isotropic.
4
5
Polycrystals
• Most crystalline solids are composed of many
small crystals (also called grains).
• Initially, small crystals (nuclei) form at various
positions.
• These have random orientations.
• The small grains grow and begin to impinge on
one another forming grain boundaries.
Micrograph of a polycrystalline
stainless steel showing grains
and grain boundaries
6
Polycrystals
• Most engineering materials are polycrystals.
Anisotropic
1 mm
• Nb-Hf-W plate with an electron beam weld.
• Each "grain" is a single crystal.
• If grains are randomly oriented,
Isotropic
overall component properties are not directional.
• Grain sizes typical range from 1 nm to 2 cm
(from a few to millions of atomic layers).
7
Single vs Polycrystals
• Single Crystals
E (diagonal) = 273 GPa
-Properties vary with
direction: anisotropic.
-Example: the modulus
of elasticity (E) in BCC iron:
E (edge) = 125 GPa
• Polycrystals
-Properties may/may not
vary with direction.
-If grains are randomly
oriented: isotropic.
200 µm
(Epoly iron = 210 GPa)
8
9
Lattice parameters in cubic, orthorhombic and
hexagonal crystal systems.
(c) 2003 Brooks/Cole Publishing / Thomson Learning™
Unit Cells Types
A unit cell is the smallest component of the crystal that reproduces the whole
crystal when stacked together.
• Primitive (P) unit cells contain only a single lattice point.
• Internal (I) unit cell contains an atom in the body center.
• Face (F) unit cell contains atoms in the all faces of the planes composing the cell.
• Centered (C) unit cell contains atoms centered on the sides of the unit cell.
Primitive
Body-Centered
Face-Centered
End-Centered
Combining 7 Crystal Classes (cubic, tetragonal, orthorhombic, hexagonal, monoclinic, triclinic,
trigonal) with 4 unit cell types (P, I, F, C) symmetry allows for only 14 types of 3-D lattice.
The fourteen
(14) types of
Bravais lattices
grouped in
seven (7)
systems.
(c) 2003 Brooks/Cole Publishing / Thomson Learning™
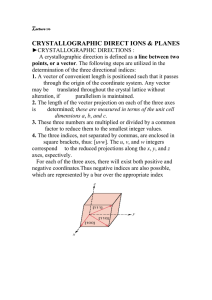
Points, Directions and Planes in the
Unit Cell
o Miller indices - A shorthand notation to describe certain
crystallographic directions and planes in a material.
Denoted by [ ], <>, ( ) brackets. A negative number is
represented by a bar over the number.
Point Coordinates
• Coordinates of selected points in the unit cell.
• The number refers to the distance from the origin in terms
of lattice parameters.
Point Coordinates
z
Point coordinates for unit cell
center are
111
c
a/2, b/2, c/2
000
a
x
y
b
Point coordinates for unit cell
corner are 111
•
z
½½½
2c
•
•
•
b
y
Translation: integer multiple of
lattice constants à identical
position in another unit cell
b
15
Crystallographic Directions
Determine the Miller indices of directions A, B, and C.
(c) 2003 Brooks/Cole Publishing /
Thomson Learning™
SOLUTION
Direction A
1. Two points are 1, 0, 0, and 0, 0, 0
2. 1, 0, 0, -0, 0, 0 = 1, 0, 0
3. No fractions to clear or integers to reduce
4. [100]
Direction B
1. Two points are 1, 1, 1 and 0, 0, 0
2. 1, 1, 1, -0, 0, 0 = 1, 1, 1
3. No fractions to clear or integers to reduce
4. [111]
Direction C
1. Two points are 0, 0, 1 and 1/2, 1, 0
2. 0, 0, 1 -1/2, 1, 0 = -1/2, -1, 1
3. 2(-1/2, -1, 1) = -1, -2, 2
4. [ 1 22]
Families of Directions <uvw>
• For some crystal structures, several
nonparallel directions with different
indices are crystallographically
equivalent; this means that atom
spacing along each direction is the
same.
18
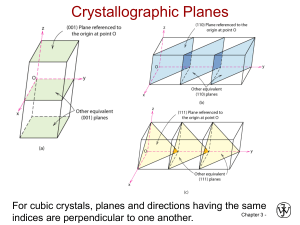
Crystallographic Planes
• If the plane passes thru origin, either:
– Construct another plane, or
– Create a new origin
– Then, for each axis, decide whether plane
intersects or parallels the axis.
• Algorithm for Miller indices
1. Read off intercepts of plane with axes in
terms of a, b, c
2. Take reciprocals of intercepts
3. Reduce to smallest integer values
4. Enclose in parentheses, no commas.
19
Crystallographic Planes
• Crystallographic planes are specified by 3 Miller
Indices (h k l). All parallel planes have same Miller
indices.
20
Crystallographic Planes
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1
1/1
1
1
4.
Miller Indices
(110)
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1/2
1/½
2
2
4.
Miller Indices
(200)
b
1
1/1
1
1
c
¥
1/¥
0
0
c
b
a
x
b
¥
1/¥
0
0
y
z
c
¥
1/¥
0
0
c
a
y
b
x
21
Crystallographic Planes
example
1. Intercepts
2. Reciprocals
3.
Reduction
4.
Miller Indices
a
1/2
1/½
2
6
b
1
1/1
1
3
c
3/4
1/¾
4/3
4
z
c
(634)
a
•
•
•
y
b
x
22
Family of Planes
• Planes that are crystallographically
equivalent have the same atomic packing.
• Also, in cubic systems only, planes having
the same indices, regardless of order and
sign, are equivalent.
• Ex: {111}
_
_
_
___
__
_ _
__
= (111), (111), (111), (111), (111), (111), (111), (111)
Ex: {100} = (100), (010), (001), (100), (010), (001)
23
FCC Unit Cell with (110) plane
24
BCC Unit Cell with (110) plane
25
SUMMARY
• Crystallographic points, directions and planes are
specified in terms of indexing schemes.
• Materials can be single crystals or polycrystalline.
• Material properties generally vary with single
crystal orientation (anisotropic), but are generally
non-directional (isotropic) in polycrystals with
randomly oriented grains.
• Some materials can have more than one crystal
structure. This is referred to as polymorphism (or
allotropy).
26