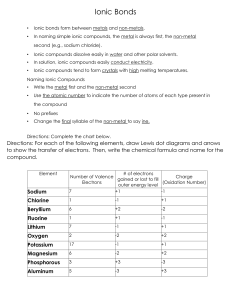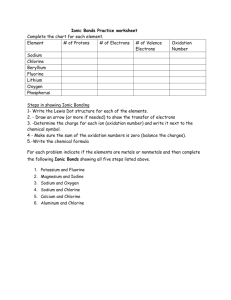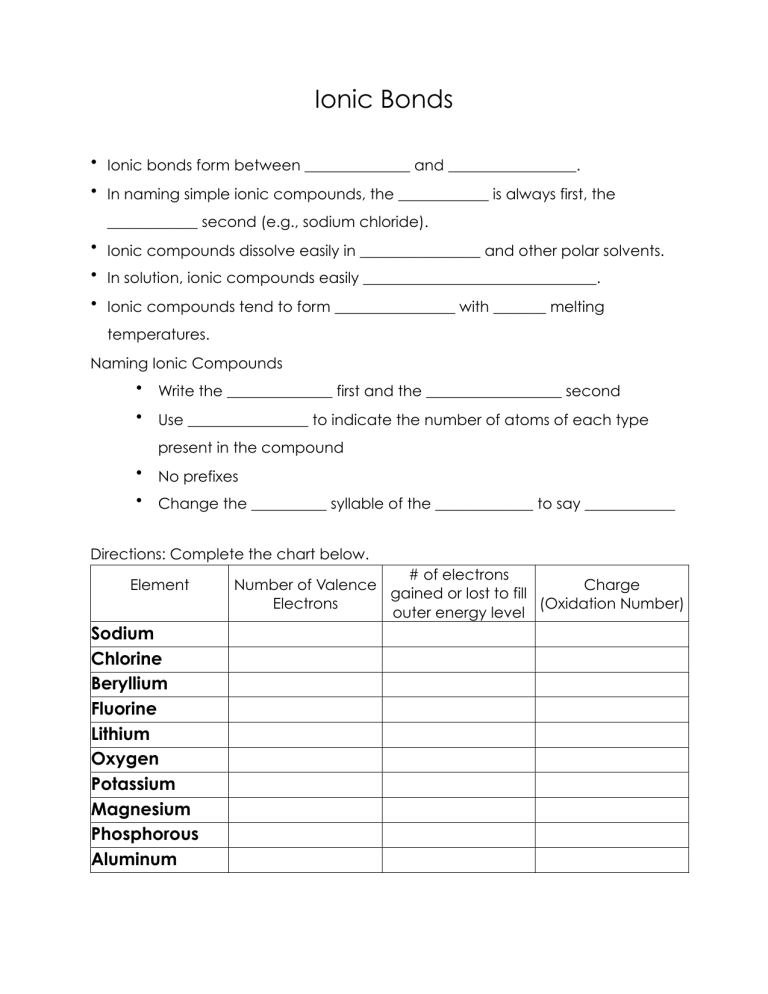
Ionic Bonds • Ionic bonds form between ______________ and _________________. • In naming simple ionic compounds, the ____________ is always first, the ____________ second (e.g., sodium chloride). • Ionic compounds dissolve easily in ________________ and other polar solvents. • In solution, ionic compounds easily _______________________________. • Ionic compounds tend to form ________________ with _______ melting temperatures. Naming Ionic Compounds • Write the ______________ first and the __________________ second • Use ________________ to indicate the number of atoms of each type present in the compound • No prefixes • Change the __________ syllable of the _____________ to say ____________ Directions: Complete the chart below. Element Sodium Chlorine Beryllium Fluorine Lithium Oxygen Potassium Magnesium Phosphorous Aluminum # of electrons Number of Valence Charge gained or lost to fill Electrons (Oxidation Number) outer energy level Directions: For each of the following elements, draw Lewis dot diagrams and arrows to show the transfer of electrons. Then, write the chemical formula and name for the compound. 1)Sodium + Chlorine 2) Potassium + Iodine Formula: _______________________ Formula: _______________________ Name: _________________________ Name: _________________________ 3) Magnesium + Oxygen 4) Calcium + Sulfur Formula: ________________________ Formula: ________________________ Name: __________________________ Name: __________________________ 5) Calcium + Chlorine 6) Magnesium + Fluorine Formula: ________________________ Formula: ________________________ Name: __________________________ Name: __________________________ 7) Potassium + Bromine 8) Potassium + Oxygen Formula: ________________________ Formula: ________________________ Name: __________________________ Name: __________________________ 9) Sodium + Oxygen 10) Aluminum + Chlorine Formula: ________________________ Formula: ________________________ Name: __________________________ Name: __________________________ 11) Calcium + Fluorine 12) Magnesium + Iodine Formula: ________________________ Formula: ________________________ Name: __________________________ Name: __________________________