#Preparation and properties of the inclusion complex of chlorohexidine with β-Cyclodextrin
advertisement

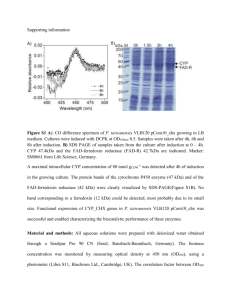
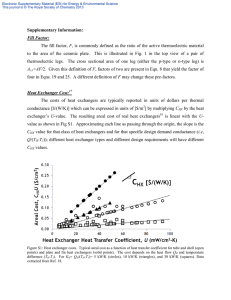
Materials Letters 309 (2022) 131460 Contents lists available at ScienceDirect Materials Letters journal homepage: www.elsevier.com/locate/matlet Preparation and properties of the inclusion complex of chlorohexidine with β-Cyclodextrin Lidi Cheng a, b, Zexian Xu a, b, Yali Li a, Dongyang Zhou a, b, Liqiang Chen a, Yanshan Liu a, Yaoxiang Xu a, Kun Meng a, b, Jian Sun a, b, c, d, * a The Affiliated Hospital of Qingdao University, Qingdao 266003, China School of Stomatology, Qingdao University, Qingdao 266003, China Dental Digital Medicine & 3D Printing Engineering Laboratory of Qingdao, Qingdao 266003, China d Shandong Provincial Key Laboratory of Digital Medicine and Computer-assisted Surgery, Qingdao 266003, China b c A R T I C L E I N F O A B S T R A C T Keywords: Biomaterials Polymeric composites Sustained release Biocompatibility Antibacterial This paper aims to construct a β-Cyclodextrin (β-CD) chlorohexidine (CHX) inclusion complex (β-CD-CHX) and to characterize its morphology, CHX release, cytotoxicity, and antimicrobial activity. β-CD-CHX was prepared by the saturated aqueous solution method. The results of SEM showed that β-CD-CHX was a fine needle-shaped crystalline particle; and the results based on the in-vitro release showed that β-CD-CHX has excellent sustained-release property. The cytotoxicity and antimicrobial activity of β-CD-CHX against different concen­ trations (5 mg/L, 10 mg/L, 20 mg/L, 40 mg/L) were observed in cytotoxicity tests and bacteriostatic ring test, respectively. The cytotoxicity assay suggested that the solvent fractions were not cytotoxic to MC3T3-E1 at concentrations up to 20 mg/L. However, the concentration of 40 mg/L had marked cytotoxicity; the β-CD-CHX at different concentrations showed excellent antibacterial effect against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). 1. Introduction large solubility, sudden release [12], difficulty in reaching lesions, and low bioavailability. The research on β-CD-CHX was carried out on the basis of the structural characteristics of β-CD and CHX. However, the cytocompati­ bility and antibacterial activity of β-CD-CHX have not been reported. Therefore, this study aims to prepare β-CD-CHX and to study its physi­ cochemical properties, in vitro release, cytocompatibility and antimi­ crobial activity. Currently, drug sustained-release systems have been widely applied in the field of pharmacology [2]. The drug release systems consist of two main components: the drug and the carrier. Therefore, the study and selection of drug and carrier materials are practical in the construction of drug sustained-release systems. CHX is a broad-spectrum antibacterial agent widely used in the field of dentistry [3]. The appropriate doses of CHX are more effective in treating patients with complicated surgical infections [4]. However, the molecular weight of CHX is 505.45, and when CHX acts directly on the infected site, the sudden release will occur, making sustained release unachievable [5]. β-Cyclodextrin (β-CD), a cyclic oligosaccharide [6,7], has a special cone-shaped structure with a hydrophobic outer surface and hydrophilic inner space, enabling it to interact with drug molecule (guest) to form inclusion complex via non-covalent bonds. By altering properties of drug molecules such as stability [8], volatility, solubility [9–11], β-CD encapsulation can overcome the limitations of drug’s action, such as 2. Materials and methods 2.1. Preparation and characterization The β-CD-CHX was prepared by the saturated aqueous solution method [1], 1.437 g β-CD was dissolved in double-distilled water; 0.64 g CHX was dissolved in 10 ml ethanol; and the molecular molar ratio of β-CD to CHX was 1:1. The morphology and elemental compositions of CHX, β-CD, and β-CD-CHX were detected by SEM (JSM-7001F, Japan) and EDS (IXRF- * Corresponding author at: The Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Qingdao 266003, China. E-mail address: sunjianqy@126.com (J. Sun). https://doi.org/10.1016/j.matlet.2021.131460 Received 18 April 2021; Received in revised form 18 November 2021; Accepted 4 December 2021 Available online 8 December 2021 0167-577X/© 2021 Elsevier B.V. All rights reserved. L. Cheng et al. Materials Letters 309 (2022) 131460 Fig. 1. (A) SEM images, (B) Element composition, (C) FTIR spectra and (D) XRD patterns of CHX, β-CD and β-CD-CHX. 550i, USA). The molecular structure and crystal phase of the three were analyzed by FTIR (Nicolet 170SX, USA) and XRD (Rigaku, Japan). (INCUCYTESS3, EssenBioscience, USA). The filter paper sheet was cut into discs with 1 cm diameter, which were then sterilized under UV light for 1 h. The discs were immersed in different concentrations of β-CD-CHX solution and attached to petri dishes coated with S. aureus (ATCC 6538) and E. coli (ATCC 8739). The antibacterial activity was determined by measuring the diameter of the inhibition zone around the filter paper disk. 2.2. In vitro release The concentrations of 4, 6, 8, 10, 12, 16, and 20 μg/mL of CHX aqueous solutions were prepared, and the absorption intensities at 255 ± 2 nm were determined by the UV–VIS spectrophotometer. The stan­ dard curve of CHX was drawn with concentration as the abscissa and absorbance as the ordinate. The absorbance values of β-CD-CHX were measured at different detection time points, the cumulative release was calculated according to the standard curve equation. The cumulative release formula is: the amount of drug released from the β-CD-CHX / amount of drug-loaded in the β-CD-CHX × 100%, 3. Results and discussion 3.1. Characterization of β-CD-CHX Under the SEM, β-CD-CHX is a fine needle-shaped crystalline particle (Fig. 1A), and its morphology is different from the irregular cuboid of β-CD. EDS results show that β-CD-CHX contains all the characteristic elements (C, N, O, Cl) of β-CD and CHX (Fig. 1B), confirming the suc­ cessful preparation of β-CD-CHX. Fig. 1C showed that the characteristic β-CD peak at 1004 cm− 1, 1209 cm− 1, 2977 cm− 1, representing the stretching vibration of C–O, –OH and C–H, respectively. The characteristic peaks of CHX at 1246 cm− 1, – N, and 1592 cm− 1, 3002 cm− 1 are the tensile vibrations of C–O–C, C– –CH2, respectively. The spectrogram of β-CD-CHX retains some char­ acteristic peaks of β-CD, such as the C–H stretching vibration peak in 2977 cm− 1 and the bending vibration of –OH at 1209 cm− 1. In addition, the peak at 3002 cm− 1 is the stretching vibration of C–H in –CH2 from CHX, and this absorption peak still exists in the spectrum of β-CD-CHX. – N at 1592 cm− 1 of CHX is weakened with a weak The absorption of C– 2.3. Cytotoxicity and antibacterial properties The MC3T3-E1 (ATCC, Rockville, MD, USA) were treated with different concentrations of β-CD-CHX (0 mg/L, 5 mg/L, 10 mg/L, 20 mg/L, 40 mg/L) after being cultured at 37 ◦ C for 24 h. After that, the α-DMEM medium was replaced, and the β-CD-CHX solution was added for co-culture for 1, 3, and 5 days. The absorbance at 450 nm was detected by the CCK-8 kit as the OD value. Each group of solutions was co-cultured with MC3T3-E1 until 80% of cells were fused, and FDA and PI working buffer were added for cell staining while avoiding light. The growth status of MC3T3-E1 in β-CDCHX solution was observed using a living cell imaging system Fig. 2. (A) The ultraviolet absorption of CHX between 200 and 400 nm, (B) The standard curve of CHX, (C) In-vitro release profiles of CHX and β-CD-CHX. 2 L. Cheng et al. Materials Letters 309 (2022) 131460 Fig. 3. (A) Fluorescence microscopy images of FDA/PI staining, (B) CCK-8 assay results for MC3T3-E1 on different concentration of β-CD-CHX (5 mg/L, 10 mg/L, 20 mg/L, 40 mg/L), (C) Photos of inhibition rings (a: S.aureus b: E. coli 1–5: 5 mg/l, 10 mg/l, 20 mg/l, 40 mg/l ), (D) Distribution of the diameter of the inhibition ring. (*P < 0.05). redshift, indicating that the benzene ring of CHX molecule enters the cavity of β-CD, and a new phase is formed between CHX and β-CD. In the aqueous solution, the size of the guest CHX molecule fits the hydro­ phobic cavity of β-CD, and the two form an encapsulant. Fig. 1D shows that diffraction peaks from the pure CHX are detected at 2θ = 12.5◦ , 17.4◦ , 19.1◦ , 21.1◦ , 22.8◦ , 24.5◦ , 26.9◦ , 34.6◦ (JCPDS No. 41-1671). The characteristic diffraction peaks of β-CD are observed at 2θ = 10.8◦ , 12.8, ◦ 15.7◦ , 18.5◦ , 20.7◦ , 22.8◦ , 27.1◦ (JCPDS No. 531903). However, the characteristic diffraction peaks of β-CD-CHX are observed at 2θ = 14.5◦ , 18.6◦ , 22.4◦ , 23.5◦ , 27.7◦ , which originally belongs to CHX β-CD still exists, while some crystalline peaks dis­ appeared. Therefore, the structure of CHX is changed significantly after its encapsulation with β-CD, confirming the results of EDS. solution, the diameter of the inhibition zone gradually increased (Fig. 3C), indicating a gradual increase in inhibition against S. aureus and E. coli (Fig. 3D). 4. Conclusion β-CD-CHX was prepared by the saturated solution method in a simple process. Then, it was characteriarized by SEM, EDS, FTIR, and XRD. In vitro release, its cytotoxicity, and antibacterial properties were analyzed. The results showed that β-CD-CHX prepared by saturated water solution method had perfect encapsulation and sustained-release effect. 20 mg/L is considered to be an ideal concentration with excellent biocompatibility and antibacterial activity. The application of β-CD-CHX in the field of drug delivery needs to be further explored. 3.2. In vitro release CRediT authorship contribution statement The Standard curve of CHX is: y = 0.04874 + 0.05374x; R2 = 0.99425 (where, y is measured absorbance of CHX; x is the concentra­ tion of CHX (Fig. 2B). The concentration of CHX contained in the release solution is found from the regression equation of the CHX standard curve. CHX can be released from the β-CD-CHX for more than 30 d, it can be seen that there is a slow-release effect on CHX (Fig. 2C). Lidi Cheng: Investigation, Data curation, Visualization, Writing – original draft. Zexian Xu: . Yali Li: Visualization, Writing – original draft. Dongyang Zhou: Software. Liqiang Chen: Conceptualization, Methodology, Resources, Writing – review & editing. Yanshan Liu: Conceptualization, Methodology, Resources, Writing – review & editing. Yaoxiang Xu: Investigation, Data curation, Software. Kun Meng: Investigation, Data curation. Jian Sun: Conceptualization, Methodol­ ogy, Resources. 3.3. Cytotoxicity and antibacterial activity The results showed that a large number of MC3T3-E1 survived in 0 mg/L, 5 mg/L, 10 mg/L, 20 mg/L aqueous solutions of β-CD-CHX and were stained green by the FDA working solution; the dead cells labeled red with PI were almost invisible; and there were a large number of dead cells labeled with red fluorescence in 40 mg/L aqueous solutions of β-CD-CHX (Fig. 3A). At the 1st, 3rd and 5th days of culture, the number of MC3T3-E1 at the concentrations of 5 mg/L, 10 mg/L, and 20 mg/L were not significantly different from that of the control group (0 mg/L) (P > 0.05). With the increase of the mass concentration of the β-CD-CHX, 40 mg/L β-CD-CHX solutions had a certain inhibitory effect on the cells (Fig. 3B). The cytotoxicity levels at the concentrations of the 5 mg/L, 10 mg/L, 20 mg/L, and 40 mg/L were evaluated according to the test standard of USP, and they grade 1, 1, 1, and 2, respectively, on days 1, 3, and 5. MC3T3-E1 could grow and proliferate in β-CD-CHX solution, and the number of cells in four groups increased with time. In addition, with the concentration increase of β-CD-CHX aqueous Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements This study was supported by the Shandong Provincial Medical and Health Science and Technology Development Plan Project (2016WS0179); Shandong Provincial Science and Technology Development Project (2014GSF118108). 3 L. Cheng et al. Materials Letters 309 (2022) 131460 References [7] M. Abbasi, J. Chin. Chem. Soc. 64 (2017) 896–917. [8] L. García-Uriostegui, H.I. Melendez-Ortiz, Guillermo Toriz, Ezequiel Delgado, Mater. Lett. 196 (2017) 26–29. [9] S. Sapte, Y. Pore, J. Pharm. Anal. 6 (2016) 3003–3306. [10] A. Heydari, A. Pardakhti, H. Sheibani, Macromol. Mater. Eng. 302 (2017) 1–13. [11] L. Chen, A.M.Q. Ahmed, Y. Deng, D. Cao, D.u. Huanhuan, J. Cui, B.-J. Lee, Q. Cao, J. Drug Delivery Sci. Technol. 54 (2019). [12] K.I.R. Teixeira, Patrícia Valente Araújo, Bernardo Ruegger Almeida Neves, German Arturo Bohorquez Mahecha, Rubé n Dario Sinisterra, Maria Esperanza Corté, Pharm. Pharm. Dev. Technol. 18 (2013) 600–608. [1] L. Ren, X. Yang, W. Guo, J. Wang, G. Chen, Polymers (Basel). 12 (2020) 1–19. [2] X. Che, J. Xue, J. Zhang, X. Yang, L. Wang, Pharm. Dev. Technol. 25 (2020) 659–665. [3] Y. Yang, Z. Xu, Y. Guo, H. Zhang, Y. Qiu, J. Li, D. Ma, Z. Li, P. Zhen, B. Liu, Z. Fan, Dent. Mater. 37 (2021) 636–647. [4] G. Allen, AORN J. 112 (2020) 421–424. [5] Barbara Sanay Inoue, Sandriele Streit, Andrea Lima dos Santos Schneider, Marcia Margarete Meier, Int. J. Biol. Macromol., 148 (2020) 1098–1108. [6] X. Liu, R. Sakthivel, C.Y. Cheng, J. Luo, L. Song, J. Wu, W. He, U. Younis, R. J. Chung, Nanoscale Res. Lett. 15 (2020). 4