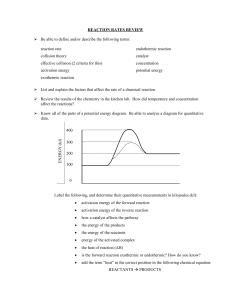
Chemical Reactions • Fireworks are a result of chemical reactions Chemical Reactions • Chemical Reactions form new substances by breaking and making chemical bonds • Chemical reactions change the way the atoms are arranged Difference between Physical and Chemical Changes • Physical • A change in the state • Solid to liquid • Liquid to gas • Gas to liquid • Atoms/molecules of the substances do not change • Chemical • Atoms/molecules broken down or combined to form new substances • Properties of new substances are different than properties of the substances that make them up Reactants and Products • Reactants: present at the beginning of the reaction • Products: are the substances formed by the chemical reaction • Example burning natural gas • CH4 + 2O2 > CO2 +2H2O • Reactants > Products Evidence of Chemical Reactions • Color Change • Formation of Precipitate (solid formed from two liquids) • Formation of gas • Temperature change: endothermic (temp down) exothermic (temp up) • Change in smell www.learner.org Classification of Chemical Reactions • Synthesis: a new compound is formed by the combination of simpler reactants • Smog formed when nitrogen and oxygen combine • N2 +2O2 > 2 NO2 oceanworld.tamu.edu Classification of Chemical Reactions • Decomposition: A reactant breaks down into simpler products (reverse of synthesis) • Water can be decomposed into hydrogen and oxygen • 2 H2O > 2H2 +O2 www.blewbury.co.uk Classification of Chemical Reactions • Combustion: One reactant is always oxygen and another reactant often contains carbon and hydrogen • Burning of methane • CH4 + 2O2 > CO2 +2H2O earthguide.ucsd.edu The Rates of Chemical Reactions can vary • Concentration: measures the number of particles present in a certain volume. • A high concentration of reactants means there is a large number of particles that can collide and react • Turning the valve on a gas stove increases the methane molecules that can combine with oxygen and results in a bigger flame and faster combustion reaction The Rates of Chemical Reactions can vary • Surface Area: the exposed surface of a substance. • To increase reaction break a large piece of the material so that there is more surface area of the material • Increase surface area=Increase reaction rate Temperature • Rate of reaction can be increased by an increase in temperature which increases how fast the particles are moving and increases the number of collisions • Remember the cold and hot water distribution of food coloring Catalyst • Catalyst: is a substance that increases the rate of chemical reaction but is not consumed in the reaction • Enzymes are catalysts that are used by living things to cause a chemical reaction. Resulting in a new product being made and the catalyst not being changed. The masses of reactants and products are equal • Law of conservation of mass: states that in a chemical reaction atoms are neither created nor destroyed. (Antoine Lavoisier) • All atoms present in the reactants are also present in the products www.iuav.it Chemical Equations • Reactants > Products • Balancing Chemical Equations • Use conservation of mass to balance: atoms can not be created or destroyed. • Reactants = products mooni.fccj.org Chemical equations • • • • • CH4 + O2 > CO2 + H2O Balanced? No Reactants: C=1, H=4, O=2 Products: C=1, H=2, O=3 H and O not equal • • • • Use coefficients to balance equations CH4 + 2O2 > CO2 + 2H2O C=1, H=4, O=4 > C=1, H= 4, O=4 Equation is now balanced! Chemical Reactions Involve Energy Changes • It takes energy to break chemical bonds. This energy is called bond energy. students.ed.uiuc.edu Exothermic Reactions • Exothermic reaction: energy is released and temp goes up…more energy is released when products are formed. • Space shuttle on take off: white clouds of water vapor are formed www.sanchezcircuit.com Exothermic Reactions www.learner.org • Exothermic reactions release energy! • All common combustion reactions are exothermic • Fireflies release energy in the form of light as an exothermic reaction Endothermic reactions • Endothermic reactions: produce a decrease in temperature because the bond energies of the reactants are greater than the bond energies of the products • All endothermic reactions absorb energy! • Alka Seltzer and water…temperature goes down. Endothermic Reactions • Photosynthesis (general formula) • 6CO2 +6H2O > C6H12O6 +6O2 • plants absorb energy from sunlight to turn carbon dioxide and water into oxygen and glucose (sugar). • Energy is stored in the glucose molecule to be used when needed. www.osovo.com Life and Industry depend on Chemical Reactions • Living things require chemical reactions • Respiration: living things get energy from glucose by respiration. (combustion of glucose) • Photosynthesis • 6CO2 +6H2O + energy> C6H12O6 +6O2 • Respiration • C6H12O6 +6O2 > 6CO2 +6H2O + energy • Respiration is the opposite of photosynthesis!