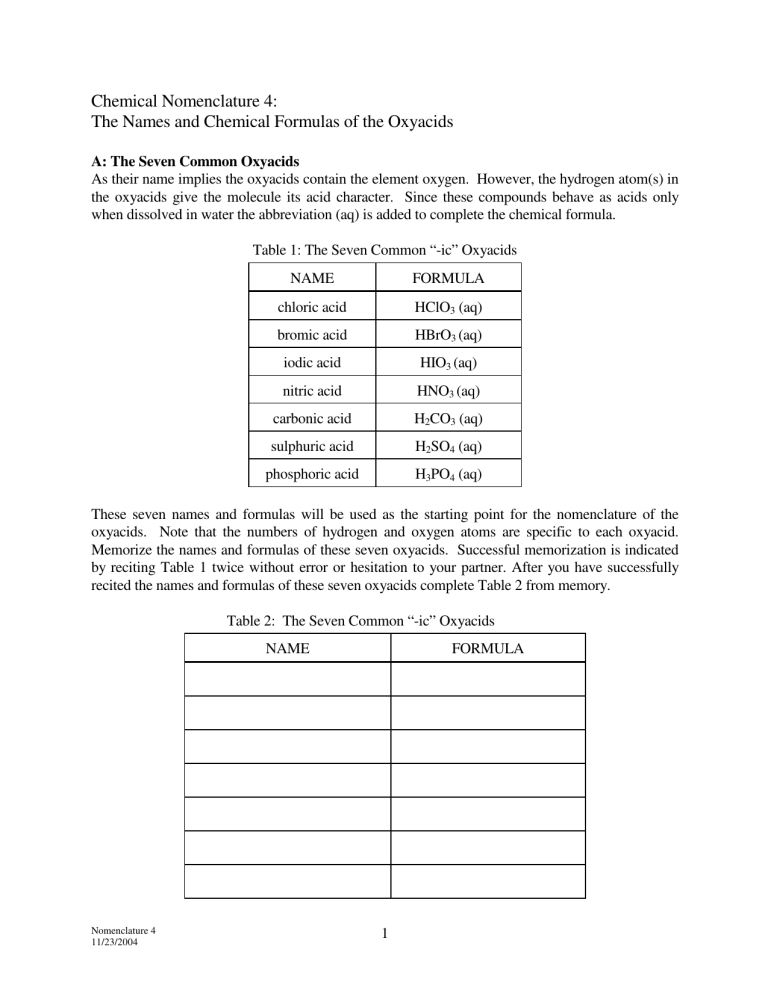
Chemical Nomenclature 4: The Names and Chemical Formulas of the Oxyacids A: The Seven Common Oxyacids As their name implies the oxyacids contain the element oxygen. However, the hydrogen atom(s) in the oxyacids give the molecule its acid character. Since these compounds behave as acids only when dissolved in water the abbreviation (aq) is added to complete the chemical formula. Table 1: The Seven Common “-ic” Oxyacids NAME FORMULA chloric acid HClO3 (aq) bromic acid HBrO3 (aq) iodic acid HIO3 (aq) nitric acid HNO3 (aq) carbonic acid H2CO3 (aq) sulphuric acid H2SO4 (aq) phosphoric acid H3PO4 (aq) These seven names and formulas will be used as the starting point for the nomenclature of the oxyacids. Note that the numbers of hydrogen and oxygen atoms are specific to each oxyacid. Memorize the names and formulas of these seven oxyacids. Successful memorization is indicated by reciting Table 1 twice without error or hesitation to your partner. After you have successfully recited the names and formulas of these seven oxyacids complete Table 2 from memory. Table 2: The Seven Common “-ic” Oxyacids NAME Nomenclature 4 11/23/2004 FORMULA 1 B: The Oxyacid Families Different oxyacids can be made by adding or reducing the number of oxygen atoms in the formulas and then re-naming the acid accordingly. There are only three variations that will be considered here. These variations apply to all of the acids listed above, but will be illustrated by example with chloric acid, HClO3 (aq). 1 Adding one oxygen atom to the formula of chloric acid (HClO3) changes the formula to HClO4. The name of the acid is altered by adding the prefix "per". The name of HClO4 (aq) is perchloric acid. 2 Removing one oxygen atom from the formula of chloric acid changes the formula to HClO2. The name is altered by replacing the "-ic" suffix with "-ous". The name of HClO2 (aq) is chlorous acid. 3 Removing two oxygen atoms from the formula of chloric acid changes the formula to HClO. The name is changed by adding the prefix "hypo-" and replacing the "-ic" suffix with "-ous". The name of HClO (aq) is hypochlorous acid. Begin by completing the names and formulas of the seven oxyacids in column #3 to Table 3 below. Use the three rules stated above to complete each cell of Table 3. Write the appropriate name if the formula is given or enter the chemical formula where the name is given. Check your work against the answers provided in Table 4. Table 3: Nomenclature of the Seven Oxyacids hypo-_______-ous acid _______-ous acid _______-ic acid per-_______-ic acid HClO4 (aq) chlorous acid HClO3 (aq) chloric acid HClO (aq) HBrO2 (aq) HBrO4 (aq) hypobromous acid bromic acid HIO (aq) HIO3 (aq) iodous acid periodic acid HNO2 (aq) HNO4 (aq) hyponitrous acid nitric acid H2CO (aq) H2CO3 (aq) carbonous acid percarbonic acid H2SO3 (aq) H2SO5 (aq) hyposulphurous acid sulphuric acid H3PO2 (aq) H3PO4(aq) phosphorous acid Nomenclature 4 11/23/2004 perphosphoric acid 2 Table 4: Complete Nomenclature of the Seven Oxyacids hypo-_______-ous _______-ous acid _______-ic acid per-_______-ic acid HClO (aq) hypochlorous acid HClO2 (aq) chlorous acid HClO3 (aq) chloric acid HClO4 (aq) perchloric acid HBrO (aq) hypobromous acid HBrO2 (aq) bromous acid HBrO3 (aq) bromic acid HBrO4 (aq) perbromic acid HIO (aq) hypoiodous acid HIO2 (aq) iodous acid HIO3 (aq) iodic acid HIO4(aq) periodic acid HNO (aq) hyponitrous acid HNO2 (aq) nitrous acid HNO3 (aq) nitric acid HNO4(aq) pernitric acid H2CO (aq) hypocarbonous acid H2CO2 (aq) carbonous acid H2CO3 (aq) carbonic acid H2CO4 (aq) percarbonic acid H2SO2 (aq) hyposulphurous acid H2SO3 (aq) sulphurous acid H2SO4 (aq) sulphuric acid H2SO5 (aq) persulphuric acid H3PO2 (aq) hypophosphorous acid H3PO3 (aq) phosphorous acid H3PO4 (aq) phosphoric acid H3PO5 (aq) perphosphoric acid acid Nomenclature 4 11/23/2004 3 1. Write the names of the oxyacids represented by the following formulas. FORMULA 2. NAME FORMULA H2SO4 (aq) HNO2 (aq) HIO2 (aq) H2CO (aq) H3PO3(aq) HBrO2 (aq) HClO2 (aq) H3PO5 (aq) HClO4 (aq) HNO (aq) H2SO3 (aq) HNO4 (aq) H2CO3 (aq) HIO3 (aq) HBrO4 (aq) HbrO (aq) H3PO2 (aq) H2SO2 (aq) HNO3 (aq) HIO4 (aq) HIO (aq) HClO (aq) H3PO4 (aq) H2CO3(aq) H2CO4 (aq) HClO3 (aq) NAME Write the formulas of the oxyacids represented by the following names. NAME FORMULA NAME perphosphoric acid chlorous acid nitrous acid sulphuric acid hypocarbonous acid iodous acid bromous acid phosphorous acid nitrous acid perchloric acid pernitric acid sulphurous acid iodic acid carbonic acid hypobromous acid perbromic acid hyposulphurous acid hypophosphorous acid chloric acid percarbonic acid phosphoric acid hypochlorous acid carbonous acid hydrosulphuric acid Nomenclature 4 11/23/2004 4 FORMULA





