Name: Date: pH Calculations We can convert any hydrogen ion
advertisement
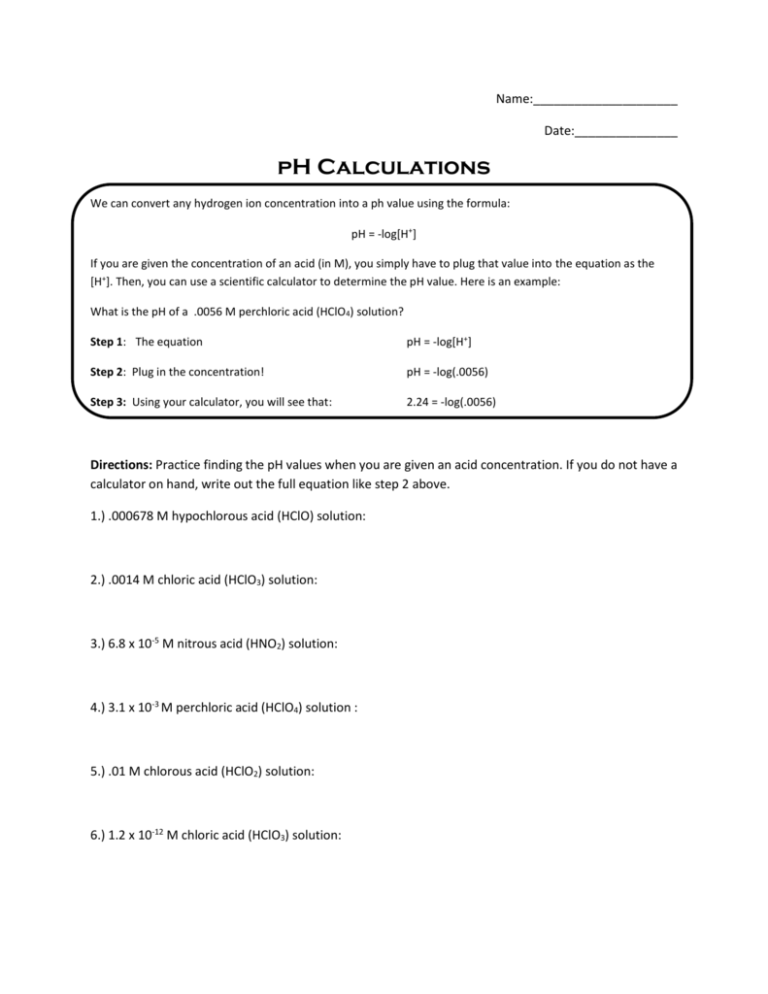
Name:_____________________ Date:_______________ pH Calculations We can convert any hydrogen ion concentration into a ph value using the formula: pH = -log[H+] If you are given the concentration of an acid (in M), you simply have to plug that value into the equation as the [H+]. Then, you can use a scientific calculator to determine the pH value. Here is an example: What is the pH of a .0056 M perchloric acid (HClO4) solution? Step 1: The equation pH = -log[H+] Step 2: Plug in the concentration! pH = -log(.0056) Step 3: Using your calculator, you will see that: 2.24 = -log(.0056) Directions: Practice finding the pH values when you are given an acid concentration. If you do not have a calculator on hand, write out the full equation like step 2 above. 1.) .000678 M hypochlorous acid (HClO) solution: 2.) .0014 M chloric acid (HClO3) solution: 3.) 6.8 x 10-5 M nitrous acid (HNO2) solution: 4.) 3.1 x 10-3 M perchloric acid (HClO4) solution : 5.) .01 M chlorous acid (HClO2) solution: 6.) 1.2 x 10-12 M chloric acid (HClO3) solution: We can also calculate the hydrogen ion concentration if we know the pH. Suppose we have a solution whose pH is 6. Its hydrogen ion concentration can be calculated as follows: Step 1: The Equation pH = -log[H+] Step 2: Plug it in 6 = -log[H+] Step 3: Move the negative sign -6 = log[H+] Step 4: Eliminate log by putting both sides to the power of 10 10-6 = 10log[H+] 10-6 = [H+] Directions: Practice finding the hydrogen ion concentration when you are given the pH of an acidic solution. You shouldn’t need a calculator to get your answer! 1.) Hypochlorous acid (HClO) solution whose pH is 3: 2.) Chloric acid (HClO3) solution whose pH is 5: 3.) Nitrous acid (HNO2) solution whose pH is 2.1: 4.) Perchloric acid (HClO4) solution whose pH is 6.89: 5.) Chlorous acid (HClO2) solution whose pH is 1.2: 6.) Chloric acid (HClO3) solution whose pH is 4.123: