1. Theories of aging:
Biological theories of aging: Some say aging is genetic, and that aging is programmed into the genetic structure. More
likely the change in our DNA and its ability to repair itself. —According to this theory, cells and tissues have vital parts
that wear out resulting in aging. —DNA undergoes continual damage throughout life, due to various reasons. DNA
damage leads to malfunctioning of genes, proteins, cells and deterioration of tissues and organs. An animal’s ability to
repair certain types of DNA damage is directly related to the life span of its species.
Most biological theories of aging have 2 general orientations:
A. Aging occurs due to random mutations and oxidative stress.
B. Aging is a result of programmed senescence.
Wear and Tear Theory: —Wilson 1974. Like a machine; our bodies simply wears out over time. —Is a programmed
process……..a biological clock that determines the maximum life span and rate at which each organ system will
deteriorate. This process is compounded by the environmental stresses (nutritional deficiencies). —Scientists have found
that the adult human brain can create new cells, opening the door to new therapies to possibly halt and even reverse
paralysis and damage from degenerative nerve disease. —Most scientists now agree that aging is, at least in part, the
result of accumulating damage to the molecules—such as proteins, lipids, and nucleic acids (DNA and RNA)—that make
up our cells. —If enough molecules are damaged, our cells will function less well, our tissues and organs will begin to
deteriorate, and eventually, our health will decline. —So in many respects, we appear to age much like a car does: Our
parts start to wear out, and we gradually lose the ability to function.
—Cellular Aging Theory: —Cellular aging is the result of a progressive decline in the proliferative capacity and life span of
cells and the effects of continuous exposure to exogenous influences that result in the progressive accumulation of
cellular and molecular damage. Suggest that cells slow their number of replications. —Cells grown in culture show a
finite number of replications. —Seems cells are programmed to follow a biological clock and stop replicating after a given
number of times. Telomeres are structures at the end of our chromosomes. —These structures shorten with each cell
division and keep track of the number of divisions a cell undergoes.
—Immunological Theory: —Defined as the declining ability of T-cells in aging organisms to replicate. T-Cells are cells of
the immune system. This theory makes use of cellular aging in that replicative senescence occurs with aging. The
declining ability of the T-cells in aging and their declining ability to replicate demonstrates that aging and a less efficient
immune system weakens the protective function and impairment in resistance to pathogens.
—Free Radical or Oxidative Stress Theory: —Free radicals -- nowadays called "reactive oxygen species" are busybody
chemicals, usually containing oxygen, that rapidly react with other chemicals. Free radicals damage proteins, DNA and
lipids, generally causing mayhem inside cells. —The presence of too many free radicals creates a condition called
"oxidative stress" in a cell. —In response, cells -- no dummies -- manufacture antioxidants. These enzymes convert free
radicals into harmless chemicals like oxygen and water. Serve to fight off attacks on our DNA by free radicals.
Mitochondrial DNA Mutation Theory:
Cross-Linkage Theory:
2. Physiologic changes with aging
Changes in body composition: —Water content of the body usually declines in the elderly, both in males and females.
Males: 60 to 54% Females: 52 to 46% Sarcopenia: = loss of muscle and fat.
—Lean body mass in muscle tissue is lost. Proportion of fat increases.
1
Changes in muscle: —Muscle fibers decrease after age 50, and muscle mass typically declines.
—Muscle tissue loses its elasticity and flexibility. Older athletes can offset this loss with vigorous exercise.
—The changes in muscle, fat and water content have serious implications for the elderly, specifically if medications are
taken.
Weight alterations: —Losses in muscle, fat and water tend to make the elderly leaner, with a lower overall body weight
and subsequent lower caloric intake. Balances in sodium and potassium also change.
—Sodium = increases by 20%. Older adults need to increase calcium, protein, and vitamin D to offset depletion.
Diet in elderly: —Many do not change their diets unless specifically advised by their provider. Other elderly eat alone,
eat poorly for several reasons: Economics Dentition = poorly fitting dentures or fractured teeth, mouth pain, loss of
salivation, and taste.
Changes in the skin: —Changes in the appearance of the skin’s texture and elasticity are the most apparent. Sun
exposure is primarily responsible for the changes in our skin known as “Photoaging” or “Extrinsic Aging”. Age spots or
“Liver spots” are harmless from a health standpoint, but worrisome to the individual.
Wound healing in elderly: —Epidermis shedding slows and cell replacement is decreased.
Dermal layer, the second layer thins because the number of dermal cells diminishes and makes it less elastic. Reduced
elasticity and thickness takes the skin longer to spring back into shape, with increased wrinkling and sagging. —
Individuals over 65 need more time to heal, as a means of closing a wound. This is one explanation for delayed epithelial
tissue replacement leading to delay in wound healing. Sebaceous and sweat glands in the dermis, deteriorate with age,
with the deepest structures losing water and fat. These changes are compounded with a decreased blood circulation to
the skin, causing change in the effectiveness in temperature regulation.
Changes in hair: —Appearance in both the hair’s thickness and appearance are evident in the elderly.Thickness
decreases by as much as 20%. We replace our hair at a rate of 60 or so hairs per day in our youth, which does not happen
as we age. This happens because we stop producing our major hormones estrogen and testosterone.
Graying of the hair: —More genetic than age. Although we are genetically predisposed to gray earlier than some, we
do lose pigment in our hair which makes the hair usually white, and gray being somewhere in between. Some elderly
have minimal changes in pigment.
Hypothermia and Hyperthermia: —Because of the alteration in the dermis, specifically blood circulation, an
older person’s comfort zone for ambient temperature is usually 4-5 degrees higher. It also takes longer for the
elder person to adjust after exposure to extreme heat or cold, leaving them more vulnerable to hypothermia
(low body temperature), and hyperthermia (heat stroke).
Changes can mean danger for the elderly: —Some drugs are processed in muscle, some in fat, and some with
water. Loss of these can lead to a retention of drugs and serious implications such as prolonged drug effect
leading to falls, disorientation, and overmedication.
3. Relocation syndrome
Relocation stress syndrome is the physical and emotional distress that occurs after the person moves from one setting to
another. Examples of physiologic behaviors are sleep disturbance and increased physical symptoms, such as GI distress.
Examples of emotional manifestations are withdrawal, anxiety, anger and depression. Family members and facility staff
need to be aware that older adults need personal space in their new surroundings. Older adults need to participate in
2
deciding how the space will be arranged and what they can keep in their new home to help offset potential feelings of
powerlessness.
Being admitted to a hospital or nursing home is often a traumatic experience. Many elders suffer from “relocation stress”
AKA relocation trauma. Physical and mental changes have been noted. In some cases, an elder who is admitted to a
hospital or SNF, can become disoriented, confused, agitated, or abusive.
Risk factors for this syndrome include:
* Lack of choice
* Lack of preparation time
* Major environmental change
4. Contributing factors to malnutrition in the elderly
Factors contributing to inadequate nutrition are:
* Inflation
* Reduced income
* Lack of transportation
* Inability to carry large quantities of groceries
Diets that consist of inappropriate or unbalanced foods may be poorly nourished
Some older adults are too proud to accept free services
Physical changes affecting nutrition: Diminished sense of taste and smell. Greater decline in ability to taste sweet and salt
than in bitter and sour. This often results in overuse of table salt and sugar
Nursing should teach patients to substitute herbs and spices to season food and vary textures to achieve satisfaction.
Tooth loss, Poorly fitting dentures. Vitamin deficiencies, constipation and other deficiencies result. The extensive use of
OTC products can also affect appetite
Some elders responds to limited mobility, diuretics and limited bladder capacity by limiting fluid intake leading to
dehydration and electrolyte imbalances.
5. Priority focus of geriatric assessment
The older person’s ability to function independently as possible is our primary concern. ADL’s and instrumental
activities of daily living. Assessing quality of life, physical mobility, self management and self efficacy, nutrition, stress
management, emotional and mental well being. Assessment is huge for depression, dementia and delirium.
6. The data collection method to elicit the most complete information during the geriatric assessment. (pg 26)
SPICES- identifies 6 serious marker conditions that can lead to longer hospital stays, higher medical costs, and even
death. It is intended to be an easy tool that has been called the “geriatric vital signs”. Use the SPICES assessment tool for
identifying serious health problems that can be prevented or managed early.
3
Sleep disorders
Problems with eating or feeding
Incontinence
Confusion
Evidence of falls
Skin Breakdown
7. The planning and implementing of nursing care for the older adult differs from that of younger adults in which
manner?
8. Major health goal of health promotion and prevention in the prevention of problems in the elderly
Healthy people 2010 set 2 major goals:
o 1. Increase quality and years of healthy life for all Americans
o 2. Eliminate health disparities among segments of the US population
o Preventative health behaviors can save the individual and society significant amounts of money for treating
advanced disease
o For example: pneumococcal vaccine to prevent pneumonia. Flu vaccine to prevent some influenza
9. Measures to help prevent drug-to-drug interactions in the elderly.
Bringing all medications to doctor's appointments, going to only one provider instead of multiple providers, having a
list of medications with you at all times, having a family member helping out to keep track of all the medications.
10. Ageism
Why has society viewed aging differently with time?
o
o
o
o
o
o
o
o
o
In the Victorian age youth was a symbol of growth and expansion. Ageism is defined as stereotyping and
discriminating against individuals or groups on the basis of their age.
The Past: Both religious and secular movements have affected the way individuals view aging.
Example: The Puritans thought the aging process was a sacred piligrimage to God.
Victorian Age: Youth was the symbol of growth and expansion.
Later: Viewed aging with sentimental indulgence or irritation.
Most people define aging in terms of ones appearance:
Visible Changes: gray hair, balding, sagging wrinkled skin, stooped shoulders, slower walk, shuffling gait.
Internal Changes: Heart, Lungs, Kidneys, and the Central Nervous System (CNS).
SENESCENCE: Biological Aging
11. Classifications of the elderly populations
Late adulthood divided into four {4} groups:
o 65 - 74 years of age: the young old
o 75 – 84 years of age: the middle old
o 85 – 99 years of age: the old old
o 100 years of age or older: the “elite” old
4
12. Nurse support interventions for the elderly feeling a “loss of control” with varying illnesses.
Nurses need to support their self esteem and feelings of independence by encouraging them to maintain as
much control as possible over their lives, to participate in decision making, and to perform as many tasks as
possible (Iggy pg 16)
13. Nutritional support for salt substitution
Remind older adults to substitute herbs and spices to season food and vary the textures of food substances to
feel satisfied. (pg 17)
Be careful with potassium levels and salt substitutes
14. Priority interventions to prevent falls in elderly patients with multiple chronic conditions.
Older persons are at the greatest risk for falls.
Falls are the leading cause of injuries and injury related deaths.
For example:
o Fractured hip, surgery risk, delayed wound healing, impaired mobility, risk for DVT (deep vein
thrombosis), physical changes required in the home, extended rehabilitation, pulmonary complications,
stasis ulcers, fat emboli.
o Slower reaction time the major cause, with associated upper body strength, causing the elderly to fall
more on their hips.
The majority of fall victims have not recovered from their pre-fall in 12 months, fall again within 6 months.
The most common causes of falls are arthritis, lack of or impaired balance control, impaired gait, cognitive
impairment, pain, medications which cause low blood pressure changes, and other Central Nervous System
(CNS) changes (fainting, dizziness, vertigo).
Most falls are preventable, through education, exercise training, change in physical home environment,
evaluation of medications.
Incapacitating accidents are a primary cause of restricted physical fitness and decreased mobility of age.
Nursing Interventions include teaching the elder about:
Safety precautions to prevent accidents such as falls
Handrails
Slip-proof underpads for rugs
Adequate lighting
Concentrate on one activity at a time
Visual and hearing assistive devices
Eye glasses, walkers, or canes
o Home modification- collaborate with family and significant others when recommending useful changes
to prevent older adult injury. Safeguards such as handrails, slip proof pads for rugs, and adequate
lighting. Avoiding scatter rugs, slippery floors, and clutter. Raised toilets seats are also important. Avoid
going out on days when steps are wet or icy and ask for help when ambulating
o Minimize sensory overload by concentrating on one activity at a time
o Changes in vision, touch, and motor ability can create challenges- Teach them to look down at where
he/she is walking and have frequent eye exams to update glasses
o Reduced sense of touch- encourage the use of visual, hearing, or ambulatory assistive devices (hearing
aids, eyeglasses, walkers)
5
o
If older adult is identified as being at high risk for falls- choose an intervention that help prevent falls. In
the community- tai chai exercise helps improve balance and functional mobility and decreases the fear
of falling. (pg 19)
15. Priority intervention for elderly patients in physical restraints.
Check the patient in a restraint every 30-60 min, and release the restraint every 2 hrs for turning, repositioning,
and toileting. If a restraint is needed, use the least restrictive device first. be sure to follow your facility’s policy
and procedure for using restraints.
use alternatives before applying any type of restraint (pg 27-28 chart 3-5)
16. Nursing interventions for agitated and combative patients (not sure if I answered this one right)***
use a calm voice to frequently reorient the patient
provide a doll or stuffed animal to “fidget” with may prevent the patient from removing important medical tubes
or equipment
provide patient with their favorite item (blanket or picture) (pg 24)
17. Age related changes for drug toxicity in the elderly.
Physiologic Changes Affecting Drug Use
* These changes affect the absorption, distribution, metabolism and excretion of drugs from the body.
* This makes drug therapy more complex and challenging
Changes that affect drug absorption from the oral route:
Increase in gastric pH
Decrease in gastric blood flow
Decrease in gastrointestinal motility
Changes that affect drug distribution:
Decreased amount of total body water
Increased ratio of adipose tissue to lean body mass {causes increased storage of lipid-soluble drugs}, leading to a
decreased concentration of the drug in plasma but an increased concentration in tissues.
Decreased albumin level
Decreased cardiac output
increased adipose tissue in proportion to lean body mass can cause increased storage of lipid soluble drugs. This
leads to a decreased concentration of the drug in plasma but an increase in tissue.
Drug metabolism often occurs in the liver:
* Result in increased plasma concentrations of drugs
Decrease in liver size
Decrease in liver blood flow
Decrease in liver enzyme activity
Decrease in renal blood flow
The excretion of drugs usually involves the renal system:
Reduced glomerular filtration rate
Decreased creatinine clearance
Slower excretion time for medications
Changes in the kidneys can also result in high plasma concentration of the drug
** Policy is to start low and go slow
6
18. Defining gradual decline in cognitive functioning
Dementia is Broad term for a syndrome that is characterized by a slowly progressive cognitive decline. Referred
to as chronic confusion
Formerly called “Organic Brain Syndrome” {OBS}.
Represents a global impairment of intellectual function and can be chronic and progressive
Types of Dementia include:
1. Alzheimer’s disease
2. Multi-infarct dementia {second most common dementia} and is a vascular disorder
19. Delirium
Defined: Is an acute state of confusion
It differs from dementia in that it is usually short-term and reversible within 3 weeks
Often seen in older adults in hospital settings or in unfamiliar settings
Behavior typically fits into two categories:
1. Hyperactivity {most common}- try to climb out of bed or become agitated, restless, and aggressive
2. Hypoactivity- quiet, apathetic and withdrawn
Multiple factors that can cause delirium:
-Drugs (esp anticholinergic and psychoactive)
- Metabolic disturbances
- Infections
- Surgical operations
- Circulatory, renal and pulmonary disorders
- Nutritional deficiencies
- Major loss
- To manage delirium, nurses should use a calm voice while reorienting the patient and try to divert attention away from
devices or tubes
20. Elder Abuse
Elders are very vulnerable to both verbal and physical abuse and neglect
Those especially vulnerable are the:
* Widowed females who may have difficulty being assertive
* Physically dependent
7
The abuser is often a family member who becomes frustrated or distraught over the burden of caring for the
elder
Assessment of Elder Abuse and Neglect
Abuse: 1) Physical- the use of physical force that results in bodily injury 2)Financial- when the older adult’s
property or resources are mismanaged 3)Emotional- the intentional use of threats, humiliation, intimidation, and
isolation toward the other adult.
o Bruises in clusters or regular patterns
o Burns (soles of the feet or buttocks)
o Unusual hair loss
o Multiple injuries (fractures)
Neglect: occurs when a caregiver fails to provide for an older adult’s basic needs, such as food, clothing,
medications, or assistance w/ ADLs. The caregiver refuses to let other people, like nursing assistance etc. into the
home
o Pressure ulcers
o Contractures, dehydration/undernutrition
o Urine burns
o Excessive body odor
o Listlessness
o bruises
o Hair loss
Reporting Abuse and Neglect
o If physical abuse or neglect is suspected, the nurse notifies the physician and social worker to investigate
the situation
o All states in the U.S. require health care professionals to report elder abuse
21. Elder Neglect- Refer to previous question
22. Government resources for the elderly
Resources for older adults
o Income: The major portion of federal funds that support the elders is devoted to the Social Security
program
o The Social Security Act was passed in 1935 to assist individuals economically impoverished following the
Great Depression
o There has been a shift from a program intended to provide minimal support to one that is the primary
source of retirement income for many elders.
o Health Insurance: Medicare is a federal health insurance program enacted as part of the amendments to
the Social Security Act of 1965.
o Provided to assist older adults to meet the cost of health care.
o Medicare has provided a means for elders to obtain needed health care in times of escalating costs
23. Interventions to reduce relocation syndrome. (Chart 3-2 pg 19)
Provide opportunities for the patient to assist in decision making
Carefully explain all procedures and routines to the patient before they occur
Ask the family or significant other to provide familiar or special keepsake to keep at bedside
Reorient the patient frequently to his or her location
Ask the patient about his/her expectations during their hospitalization stay
Encourage family and friends to visit
Establish a trusting relationship w/ pt as early as possible
Assess patient’s usual lifestyle and daily activities (food likes/dislikes, preferred time for bathing)
8
Avoid unnecessary room changes
Have family member or staff member accompany patient when leaving the unit for special procedures
24. Conditions predisposing the elderly person to increased risk of falls. (See #14)
Slower reaction time the major cause, with associated upper body strength, causing the elderly to fall more on
their hips.
The most common causes of falls are arthritis, lack of or impaired balance control, impaired gait, cognitive
impairment, pain, medications which cause low blood pressure changes, and other Central Nervous System
(CNS) changes (fainting, dizziness, vertigo).
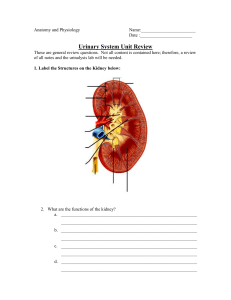
25. Patients at greatest risk for developing postrenal acute renal failure (a blockage in the bladder will back urine up
into the kidney causing damage to the nephrons)
Pts who have obstruction of the urine collecting system anywhere from calyces to the urethral meatus such as:
ureter, bladder, or urethral cancer; kidney, ureter, or bladder stones; tumors; bladder atony; prostatic hyperplasia
or cancer; urethral stricture; cervical cancer.
o Benign prostatic hyperplasia
o Prostate cancer
o Urinary caluli
o Renal tumors
o Renal trauma
o Hydronephrosis
Patho: Bilateral obstruction of urine outflow. Rapid rise in proximal tubular pressure causes the inability to concentrate or
acidify urine normally.
Patients at risk for developing intrarenal failure (Acute tubular nephrosis- damage to the nephrons)
o Kidney ischemia- decreased blood flow
o Toxic meds- abx, iodine contrasts media, NSAIDs
o Muscle trauma- Rhabdomylosis (myoglobin released from skeletal muscle into bloodstream)
o Transfusion reaction
o Systemic lupus erythematosus
o glomerulonephritis
Patients at risk for developing prerenal failure (factors outside of the kidneys)
o Dehydration
o Decreased CO
o Renal Artery narrowing
o Shock- shunt blood away from the kidneys
26. Drugs that predispose the elderly to acute renal failure.
Prerenal failure is caused by overuse of drugs such as diuretics and antihypertensive.
ARF:
o aminoglycosides (antibiotics)
o anti-fungal
o Cox 1 and Cox2 Inhibitors also known as NSAID’s (ibuprofen, aspirin and naproxen) Cox1 and 2 are
receptors in the heart, GI, and kidneys that produce prostaglandins. prostaglandins keep arterioles open.
NSAIDS block this effect, they constrict the renal arterioles→ then we see a rise in creatinine. Taking
NSAIDS without adequate hydration will cause problems
27. Fluid restriction guide for patients in acute renal failure
9
o
Maintaining fluid volume: BP, Body weight, CVP, Electrolytes are normal/near normal
Interventions: Monitor I/O, looks for S/S of fluid overload for example crackles, edema, JVD) Admin diuretics to
control BP and urinary retention. Daily weights (1kg of weight= 1L of fluid retained)
Fluid restrictions: w/ hemodialysis: 500-700 mL/day, w/chronic uremia: depends on urine output 1500-3000, w/
peritoneal dialysis: based on weight and bp
28. Assessment findings in acute renal failure
Renal: oliguria/anuria, increased urine specific gravity
Cardiac =: Hypotension, tachycardia, jugular venous distention, increased central venous pressure, ECG changes:
tall T waves, HTN, flat neck veins (low or no JVP)
Respiratory: SOB, orthopnea, crackles, pulmonary edema, friction rub
GI symp: anorexia, nausea, vomiting, and flank pain
Neuro: lethargy, HA, tremors, confusion, seizure, coma
General: generalized edema, weight gain, decreased urine output, dry mucous membranes, cool clammy skin,
infection, hematuria, petechiae, ecchymoses
(bolded were either found in both the book or slide or just the slides, the rest are from the book)
29. BUN to Creatinine ratio
Usually between 10:1 and 20:1.
An increased would indicate a decrease in blood flow to the kidneys. This may be due to CHF, urinary tract
obstruction or dehydration. Bun to Creatinine ratio is useful in differential diagnosis of acute/ chronic renal
disease. When the two levels rise and the ratio between them remains constant, it indicates kidney dysfunction.
A rise in creat is indicative of kidney damage, BUN is not always indicative unless it goes hand in hand with creat.
Asked us to know normal values of BUN, Creat and GFR during lecture
Normal Values:
o BUN: 10-20mg/dL; May be slightly increased in older adults. In patients w/AKI it may reach 180-200
mg/dL before symptoms develop.
o Creat 0.5-1.1mg/dL women, 0.6-1.2mg/dL male. In acute renal injury increases by 1-2mg/dL every 24-48
hours.
o GFR 90-120 (older patients will have a lower than normal GFR)
o Spec Grav 1.005-1.030
Creatinine Clearance- is a calculated measure of glomerular filtration rate. It is the best indication of overall
kidney function. Serum creatinine is produced when protein or muscle breaks down. Creatinine is filtered by the
kidneys and excreted in the urine. No common pathologic condition other than kidney disease increases the
serum creatinine level.
Blood Urea Nitrogen- measure the kidney excretion of urea nitrogen, a by-product of protein breakdown in the
liver. BUN levels indicate the extent of kidney clearance of this nitrogen waste product.
Specific Gravity- the density of urine compared with water. Density is related to the number of particles in a
specific volume of urine. In kidney dz, changes in spec grav do not reflect systemic volume. An increase in spec
grav occurs w/ dehydration, decreased kidney blood flow, or presence of ADH. In these situations, the normal
kidney response is to reabsorb water and decrease urine output. As a result, the urine produce is more
concentrated. Decrease occurs w/ increased fluid intake, diuretic drugs, and diabetes insipidus. In these
conditions, the normal kidney response is to excrete more water; thus urine output is increased.
30. Serum and urine osmolarity
10
Measures concentration of particles in a solution (electrolytes, glucose, urea, and creatinine)
Urine osmolarity Varies from 50 to 1400 mOsm/L, dependent on pt.’s hydration status and kidney function
Avg. urine osmolarity is 300 – 900 mOsm/L
Normal serum osmolarity is between 270-300 mOsm/L
Obligatory solute secretion: particles must be excreted in urine on regular basis
Increased Osmolarity = concentrated urine w/ less water & more solutes
Decreased Osmolarity = dilute urine with more water & fewer solutes
Prerenal AKI- urine osmolarity is decreased
Postrenal AKI- urine osmolarity is increased
Mannitol is administered through a filter in the IV tubing to eliminate microscopic crystals
Bladder catheters are usually inserted to maintain strict output
The patient’s serum and urine osmolarity are assessed daily and mannitol is used to obtain a serum osmolarity of
310-320 mOsm/L depending on the provider’s goal of therapy
31. Pathophysiologic process of acute renal failure
AKI is a rapid decrease in kidney function. It leads to the collection of metabolic wastes in the body. This
condition can result from other conditions that reduce blood flow to the kidneys also known as (prerenal acute
kidney injury). AKI may lead to ESRF but the acute stage may be reversible. Reduced blood flow (poor perfusion)
d/t toxins, tubular ischemia, infections, and obstruction have different effects on the kidney and its function. Any
of these processes can reduce the GFR, damage the nephron cells and obstruct urine flow in the kidney tubules.
The kidneys compensate by constricting the renal blood cells, reactive renin-angiotensin-aldosterone pathway
and releasing ADH. This increased blood volume and increases perfusion to the kidneys, however, this causes the
patient to have oliguria (urine output <400ml/day), azotemia (build up of nitrogen waste in blood). This causes
damage to the nephrons, which may result in ischemia, and the buildup of toxins that may lead to reduced
kidney b/f and ischemia.
When caused by infection, drugs or cancer it causes immune mediated changes in the kidney tissue. The tubular
cells slough and combine with other formed elements (RBC; casts) causing an obstruction. The pressure in the
kidney tubules exceeds glomerular pressure causing glomerular filtration to stop causing BUN and creatinine
collects in the blood. When BUN rises faster than serum creat the cause is usually r/t protein breakdown or
dehydration. When the two levels rise and the ratio between them remains constant, it indicates kidney
dysfunction. Takes less than 3 months to develop.
Nursing priority is preventing volume depletion because this is the most common cause of AKI
11
32. Interventions to reverse prerenal azotemia
Can be reversed by establishing normal intravascular volume, B/P, cardiac output. Prolonged hypoperfusion can
lead to ischemic injury and failure. Treat underlying cause hypovolemic shock/CHF, correct blood volume (blood
transfusion, IV bolus, erythropoietin)
Infusion of IV fluids: hypotonic solutions 0.5% NS, D5W
Increase BP ( IV bolus)
Encourage pts to drink as much free water as they can or admin via NG tube.
To increase CO might require careful use of diuretics, ACE’s, beta blockers, nitrates, positive inotropic agents
(dobutamine)
Improve CO (digoxin)
Fluid challenge/ diuretics: Pt with fluid overload 500-1000 mL NS infused over 1hr. Pt responds by producing
urine soon after initial bolus. Diuretics (furosemide) may be prescribed
33. Interventions for hyperkalemia
When oliguria develops, potassium is not excreted and hyperkalemia occurs when levels exceed 5.4 mEq/L
Priority Interventions: Monitor for cardiac complications (EKG, ECG)
Patient safety for falls prevention
Drug Therapy: Potassium excreting diuretics (furosemide/Lasix),
Pt w/kidney problems administer sodium polystyrene sulfonate/Kayexalate
IV Insulin Increased Na-K pumps moves K from ECF to cells
Monitor Response to therapy
34. Clinical manifestations in acute renal failure
Azotemia: buildup of nitrogenous wastes
Prerenal azotemia: Hypotenionts/tachy, decrease CO and CVP, lethargy
Intrarenal/Post renal Azotemia (damage to tissues: oliguria/ anuria, edema/htn/tachy/JVD, SOB/crackles, N/V
35. Acute tubular necrosis
Complication after kidney transplant
Results from hypoxic damage when transplantation is delayed, after kidneys have been harvested
Pts. may need dialysis until adequate urine output returns, and BUN/Creat. Normalizes
ATN is hard to distinguish between rejection -> weekly biopsies
36. Sodium bicarbonate indications and expected effects
Metabolic acidosis (chronic)
Chronic metabolic acidosis
Diarrhea
Alkalinizing agent by releasing bicarbonate ions
Indigestion
Antacid-neutralization of gastric acid
Toxicity of drug
Raises blood pH (alkaline)
Cardiac arrest d/t hyperkalemia
Diabetic Ketoacidosis
Increases urinary pH, frees bicarb ions, alkaline urine may breakdown stones
12
37. Phases of acute renal failure
Onset:
Hours to days
Begins with precipitating event
Oliguria
BUN/Creat increasing
Oliguric Phase:
Lasts 1-3 weeks
Urine output 100-400 mL/24hr
BUN/Creat increase, hyperK, bicarb deficit (met. Acidosis),
hyperphosphatemia, hypocalcemia, hypermagnesemia, hyperkalemia
Diuretics Phase (high-output)
Sudden onset; 2-6 weeks
Urine flow increases steadily, dilute unable to concentrate urine
Excessive urine output indicates that damaged nephrons are recovering their ability to excrete wastes
Diuresis can result upto 10L/day urine output
BUN/Creat decline, hyponatremia (confusion, muscle weakness), hypotension, hypokalemia
Normal Function returns
Recovery Phase (Convalescent)
May take up to 12 months
Pt. returns to normal levels of activity
Pt. may have residual kidney dysfunction
Phase
Description
Characteristics
Onset phase
Begins w/the precipitating event and continues until
oliguria develops; lasts hrs-days
gradual accumulation of nitrogenous wastes, like
increasing serum creatinine and BUN, may be noted
13
Oliguric phase
characterized by a urine output of 100-400 mL/24 hr
that does not respond to fluid challenges or diuretics;
lasts 1-3 wks
Laboratory date include increasing serum creatinine and
BUN levels, hyperkalemia, bicarbonate deficit
(metabolic acidosis), hyperphosphatemia,
hypocalcemia, & hypermagnesemia.
Sodium retention occurs, but masked by dilutional
effects of water retention.
Urine specific gravity and urine osmolarity do not vary
as plasma osmolarity changes
Diuretic phase
(high-output
phase)
Often has a sudden onset w/in 2-6 wks after oliguric
stage; urine flow increases rapidly over a period of
several days; the diuresis can result in an output of up
to 10L/day of dilute urine
A. Early Diuretic Stage: daily output > 400
mL/day to the time BUN concentration stops
rising
B. Late diuretic or Recovery stage: extends from
the day the BUN falls to stabilization –BUN stops
rising and starts to fall
Electrolyte losses typically precede clearance of
nitrogenous wastes.
Later in the diuretic phase, the BUN levels starts to fall
and continues to fall until the level reaches normal
limits or reaches a plateau.
Normal kidney tubular function is re-established during
this phase.
Recovery phase
(convalescent
phase)
In this phase, pt begins to return to normal levels of
activity. Complete recovery may take up to 12 months.
-from day BUN is stable to when the pt returns to
normal activity and urine volume and BUN is normal
Pt functions at a lower energy level and has less stamina
than before the illness.
Residual kidney dysfunction may be noted through
regular monitoring of kidney dysfunction.
Kidney function may never return to pre-illness levels,
but function sufficient for a long and healthy life is
likely.
The client with ARF has a serum K level of 6.0 mEq/L. The nurse would plan which of the following as a priority
action?
o Place the client on a cardiac monitor (Normal range 3.5-5 mEq/L)
38. Clinical manifestations for patients with a fracture to the temporal bone
- The spectrum of temporal bone trauma is extremely varied, ranging from minor concussion without functional benefits
to severe blunt or penetrating trauma with malfunctional deficits that involve the auditory and vestibular nerves, the
facial nerve, and intracranial contents. The clinical presentations specifically related to temporal bone trauma include
facial nerve paralysis (partial or complete), hearing loss (conductive, sensorineural, or mixed), vertigo, dizziness,
otorrhagia (hemorrhage from the ear), cerebrospinal spinal fluid (CSF), otorrhea (discharge from external ear), tympanic
membrane perforation, and hemotympanum (the presence of blood in the tympanic cavity of the middle ear), and canal
laceration
- Epidural hematomas
- Results from arterial bleeding into the space between the dura and the inner table of the skull.
- Often caused by a fracture of the temporal bone, which houses the middle meningeal artery.
- Epidural hematomas may be characterized by the presence of a “lucid interval” lasting for minutes, during which
the patient will be alert and talking followed by momentary unconsciousness occurring within minutes of the
injury.
- Within minutes, a potentially critical catastrophic elevation in ICP and structural changes.
- The temporal lobe process hearing, memory and language functions
- Auditory center for sound interpretation, complicated memory patterns, Wernicke’s area for language (allows
processing of words into coherent thought and understanding of written or spoken words.
39. Pathological process causing brain damage by increasing ICP
14
the normal level of ICP is 10-15mmHg
Increased ICP is the leading cause of death of head trauma in pts who arrive to the hospital alive.
Compliance can no longer take place and the brain cannot accommodate further volume changes.
As ICP increases→ cerebral perfusion decreases → tissue hypoxia, decrease in serum pH level, and increase in
level of carbon dioxide→ causes cerebral vasodilation, edema, and a further increase in ICP, and the cycle
continues. If untreated, brain may herniate downward towards the brainstem or laterally from a unilateral lesion
within one cerebral hemisphere, causing irreversible damage and possibly death.
40. Glasgow Coma Scale
o
o
o
o
o
o
o
o
o
Helps describe the patient’s LOC
Developed for assessment of patients in coma
A score of 7 represents comatose state
The lower the score, the lower the level of consciousness
Consists of three categories: • Eye opening • Best verbal • Best motor
Scores range from 3 to 15. The lower the score, the worse the patient and prognosis.
Mild TBI- GCS score of 13-15 and a loss of consciousness for 0-15 min
Moderate TBI- GCS score of 9 – 12, loss of consciousness for up to 6 hours; and may be accompanied by other
systemic injury
Severe injury- GCS of 3 – 8 and a LOC greater than 6 hours, is more serious and requires management in critical
care with ongoing monitoring of multiple physiologic parameters.
41. Cranial nerve testing
In the unconscious patient, additional oculocephalic and oculovestibular tests are performed to test the integrity of the
brainstem and of the CNs III, VI, and VII.
Cranial Nerve I
- Olfactory (sensory)
- Smell
- Usually not tested
- May be tested in patients after head injury or pituitary surgery
- Use coffee for testing
Cranial Nerve II Optic
- Optic (sensory)
- Vision
- Visual acuity (read badge)
- Visual fields (confrontation)
15
- Fundoscopic examination (papilledema, disk atrophy, retinal hemorrhages, corneal scarring, cataracts)
Cranial Nerves III Oculomotor, IV Trochlear and VI Abducens
- Oculomotor, trochlear, abducens
- Motor nerves that are tested together
- Eye movement
- Eyelid opening
- Pupil reaction
Cranial Nerve V
- Trigeminal nerve (mixed)
- Sensory component
o 3 divisions - Test with light touch, pinprick, and temperature
o Corneal reflex- Direct and consensual eye blink
Cranial nerve VII is the motor component of the reflex arc
o Motor component- Muscles of mastication
Temporalis
Masseter- Tested by having patient clench teeth
Cranial Nerve VII Facial
- Facial (mixed)
o Sensory component
Taste anterior 2/3 of tongue
o Motor component
Innervation of the face
Cranial Nerve VIII Vestibulocochlear
- Acoustic (sensory)
o Cochlear component (hearing)
Rubbing fingers or whisper into ear
Vestibular component (balance)
Nystagmus and vertigo
Cold caloric testing (coma vs. awake)
Cranial Nerve IX Glossopharyngeal and X Vagus
- Tested together due to overlap in function
- Sensory
o Posterior pharynx and larynx
o Motor
Soft palate, pharyngeal muscles, vocal cords
o Phonation and gag reflex
Cranial Nerve XI Accessory
- Spinal accessory (motor)
Sternocleidomastoid and trapezius muscles
o Shoulder shrug
o Rotate head against resistance
Cranial Nerve XII Hypoglossal
- Hypoglossal (motor)
o Observe tongue at rest
o Tongue protrusion
o Lateral pressure to each cheek
16
43. Spinal Shock
aka neurogenic shock: loss of sensation accompanied by motor paralysis with initial loss but gradual recovery of reflexed,
following spinal cord injury- most often a complete transection.
occurs immediately as the cord’s response to injury
complete but temporary loss of motor, sensory, reflex, autonomic function could last 48 hrs- weeks
there is a disruption between upper/lower motor neurons
characterized by: flaccid paralysis, loss of reflex activity below level of lesion, bradycardia, paralytics ileus, and
hypotension
neurogenic bladder or no bladder tone (indwelling catheter for distention)
may last from a few days to a few months, reversal is indicated by return of reflex activity
reflexes in the spinal cord caudal (posterior part of body) to the SCI are depressed (hyporeflexia) or absent
(areflexia), while those rostral (front part of body) the SCI are unaffected
muscle spasticity, reflex activity, and bladder function begin in pts w/ cervical or high thoracic injuries when
spinal shock is resolved
17
44. Patients at greatest risk for Autonomic Dysreflexia
- Patients with spinal cord injuries at Thoracic 5 (T-5) level and above are very susceptible
Pathophysiology-(A) A strong sensory input (not necessarily noxious) is carried into the spinal cord via intact peripheral nerves.
The most common origins are bladder and bowel.
(B) This strong sensory input travels up the spinal cord and evokes a massive reflex sympathetic surge from the
thoracolumbar sympathetic nerves, causing widespread vasoconstriction, most significantly in the
subdiaphragmatic (or splanchnic) vasculature. Thus, peripheral arterial hypertension occurs.
(C) The brain detects this hypertensive crisis through intact baroreceptors in the neck delivered to the brain
through cranial nerves IX and X.
(D) The brain attempts two maneuvers to halt the progression of this hypertensive crisis.
First, the brain attempts to shut down the sympathetic surge by sending descending inhibitory impulses.
These impulses are unable to travel to most sympathetic outflow levels because of the spinal cord injury
at T6 or above. Inhibitory impulses are blocked in the injured spinal cord.
In the second maneuver, the brain attempts to bring down peripheral blood pressure by slowing the heart
rate through an intact vagus (parasympathetic) nerve; however, this compensatory bradycardia is
inadequate and hypertension continues. In summary, the sympathetics prevail below the level of
neurologic injury, and the parasympathetic nerves prevail above the level of injury. Once the inciting
stimulus is removed, reflex hypertension resolves.
etiologies: most common cause is overfilling of bladder, second most common cause is a bowel that is full of
stool or gas, any stimulus to the rectum (ex:digital stimulation), skin irritations, wounds, pressure sores, burns,
broken bones, pregnancy, ingrown toenails, appendicitis, and other medical complications
collaborative interventions.: treatment was be initiated quickly, place pt in sitting position (first priority!),
notify HCP, loosen tight clothing, assess for and treat the cause, check the urinary catheter tubing (if present) for
kinks or obstruction, check for bladder distention and catherize quickly if present (place anesthetic ointment on tip
before insertion), check pt for fecal impaction (disimpact immediately using anesthetic ointment), check the room
temp to ensure that it is not too cool or drafty, monitor BP Q10-15 min, give nitrates or hydralazine as prescribed.
(anti-HTN)
A sudden increase in sympathetic nervous system stimulation can occur after spinal shock resolves. A stimulus,
such as urine flow obstruction or constipation stimulates the SNS to release epinephrine and NE. The pt will have
18
a sudden and severe elevation in BP, pounding headache, profuse sweating and goosebumps above the level of
injury. The pt is at risk for stroke if the BP does not immediately decrease.
45. Treatment for spasticity in patients with Spinal Cord injuries.
intrathecal baclofen (Lioresal) therapy- skeletal muscle relaxant that is administered through a programmable,
implantable infusion pump and intrathecal catheter directly into the CSF. thr pump is surgically placed in a
subcutaneous pouch in the lower abdomen. Monitor for adverse effects: sedation, fatigue, dizziness, and
changes in mental status. (seizures and hallucinations may occur if suddenly stopped.
tizanidine- may help control spasticity, however, they cause severe drowsiness and sedation in most patients and
may not be effective in reducing spasticity. baclofen is the best alternative.
46. Gardner Wells tongs
- Shaped spinal tongs used for spinal traction for surgical spinal cord injuries. Controlled pins are inserted into the skull
at opposite ends to permit application of a longitudinal force to the axis of the spinal column. Purpose is to maintain
alignment and reduce injury. Major complication is that pt are at risk for the complications of immobility, and infection
Maintain the line of pull
Keep weights hanging freely
Maintain traction at all times
Monitor pin sites for inflammation and infection
If tong becomes displaced, hold pts head in neutral position until tong can be repositioned
47. Clinical manifestations of increasing ICP
19
As ICP increases, cerebral perfusion decreases, leading to tissue hypoxia, a decrease in serum pH level, and an increase in
CO2.
Early signs: headache and changes in level of consciousness
pupils may become sluggish, unequal, dilated, and nonresponsive to light, temporal field blindness
Late sign- Cushing’s Triad: increased systolic blood pressure, widened pulse pressure, bradycardia, and abnormal
respiratory pattern
headache, vomiting, drowsiness, and lethargy
The most important short-term goal for a client with increased ICP is controlling agitation and restlessness
48. Basilar skull fractures, clinical manifestations, collaborative management
- If unrecognized and untreated can be fatal.
- Open head fracture that occurs at the base of the skull, usually extending into the anterior, middle, or posterior fossa
resulting in leakage of CSF from the nose or ears.
clinical manifestations: Racoon eyes- bilateral periorbital ecchymosis, leakage of blood or CSF from ears,
abnormalities in pupillary response, battle’s sign seen several days after (discoloration behind the ear), potential
for hemorrhage caused by damage to the internal carotid artery, damage to CN I, II, VII, and VIII, and infection.
- Manifestations:
o Raccoon eyes = bilateral periorbital ecchymosis
o Blood or CSF from ears
o Battle's sign is seen several days following a basilar skull fracture. There may have been bloody drainage
from the ear immediately after the fracture occurred.
o A discoloration behind the ear in the line of the posterior auricular artery, often associated with a basilar
skull fracture.
- Interventions: Place the patient supine with the HOB elevated 30 degrees. Monitor neurologic signs. If the
patient is found to have a large dural tear, surgery is indicated.
- keep patient on complete bedrest, frequent neuro checks. If unrecognized and untreated can be fatal
- occurs at the base of the skull, usually extending into the anterior, middle, or posterior fossa resulting in leakage
of CFS from the nose or ears.
20
49. Pupillary changes with increasing ICP
-
-
-
Basilar skull fracture and other head injuries may damage cranial nerve III, the oculomotor nerve.
In that event, the patient will exhibit abnormalities of pupillary response, such as paralysis of light reflex, and
other functional disturbances of sight. Pupillary dilation (mydriasis) indicates unopposed sympathetic activity
due to impaired parasympathetic axons.
This may reflect compression or distortion of the oculomotor nerve by either primary injury or herniation.
Mydriasis also may be an effect of adrenergic stimuli such as epinephrine, anticholinergics, cocaine, PCP, and
drug withdrawal.
The classic fixed and dilated "blown pupil" is a unilateral phenomenon that may occur when a rapidly expanding
intracranial mass, including blood from a hemorrhage, is compressing cranial nerve III. It may also represent
herniation of the uncus of the temporal lobe.
Pupils that are fixed (nonreactive) and dilated are a poor prognostic sign, resulting from marked increase ICP.
Patients with this problem are sometimes referred to as having “blown” pupils
pupils may become sluggish, unequal, dilated, and nonresponsive to light, temporal field blindness
management: compare pupil size, shape and equality bilaterally
o check pupils with direct eye reflex (check each eye individually)
o check PERRLA (pupils equal, round, reactive to light
o assess 6 cardinal fields of gaze (cranial nerves 3, 4, and 6)
o
assess for Doll’s eye phenomenon in unconscious patients (indicates brain stem damage)
50. Education for the patients with head injury and functioning
instruct pt and family should be instructed to return to the hospital if any of these problems occur:
o fever greater than 100ºF, pulses <50 bpm, vomiting, slurred speech, dizziness, blurred or double vision,
unequal pupil size, blood or fluid discharge from ears or nose, increased sleepiness, inability to move
extremities, convulsions, unconsciousness
21
51. Osmitrol (Mannitol)
C: osmotic diuretic
Indication: IV: Adjunct in the treatment of: Acute oliguric renal failure, Edema, Increased intracranial or
intraocular pressure, Toxic overdose; GU irritant: During transurethral procedures
Action: Increases the osmotic pressure of the glomerular filtrate, thereby inhibiting reabsorption of water and
electrolytes. Causes excretion of: Water, Sodium, Potassium, Chloride, Calcium, Phosphorus, Magnesium, Urea,
Uric acid.
Therapeutic Effect(s): Mobilization of excess fluid in oliguric renal failure or edema. Reduction of intraocular or
intracranial pressure. Increased urinary excretion of toxic materials. Decreased hemolysis when used as an
irrigant after transurethral prostatic resection.
Contraindicated in: Hypersensitivity; Anuria; Dehydration; Active intracranial bleeding; Severe pulmonary edema
or congestion.
Adverse Effects: CNS: confusion, headache. EENT: blurred vision, rhinitis. CV: transient volume expansion, chest
pain, HF, pulmonary edema, tachycardia. GI: nausea, thirst, vomiting. GU: renal failure, urinary retention. F and E:
dehydration, hyperkalemia, hypernatremia, hypokalemia, hyponatremia. Local: phlebitis at IV site
Assessment: Monitor vital signs, urine output, CVP, and pulmonary artery pressures (PAP) before and hourly
throughout administration. Assess patient for signs and symptoms of dehydration (decreased skin turgor, fever,
dry skin and mucous membranes, thirst) or signs of fluid overload (increased CVP, dyspnea, rales/crackles,
edema). Assess patient for anorexia, muscle weakness, numbness, tingling, paresthesia, confusion, and excessive
thirst. Report signs of electrolyte imbalance. Increased Intracranial Pressure: Monitor neurologic status and
intracranial pressure readings in patients receiving this medication to decrease cerebral edema. Monitor for
persistent or increased eye pain or decreased visual acuity. Lab Test Considerations: Renal function and serum
electrolytes should be monitored routinely throughout therapy.
Implementation: Observe infusion site frequently for infiltration. Extravasation may cause tissue irritation and
necrosis. Do not administer electrolyte-free mannitol solution with blood. If blood must be administered
simultaneously with mannitol, add at least 20 mEq NaCl to each liter of mannitol. Confer with health care
professional regarding placement of an indwelling Foley catheter (except when used to decrease intraocular
pressure). Oliguria: Administration rate should be titrated to produce a urine output of 30–50 mL/hr.
22
52. Clinical rationale for and interventions for periorbital edema, periorbital ecchymoses, and Battle sign.
Periorbital edema and ecchymoses of one or both eyes are not unusual and are treated with cold compresses to
decrease swelling (FIRST report to HCP). Irrigate the affected eye with warm saline solution or artificial tears to
improve pt comfort.
Periorbital ecchymoses: (Racoon’s eyes/black eye) may occur following surgery and may indicate a meningeal
tear and bleeding into the sinuses.
o Contact surgeon stat! Keep pt on complete bedrest. Frequent neuro checks
Battle sign: seen several days following a basilar skull fracture. There may have been bloody drainage from the
ear immediately after the fracture occurred. A discoloration behind the ear in the line of the posterior auricular
artery, often associated with a basilar skull fracture.
o if unrecognized and untreated can be fatal
o place patient supine w/ HOB elevated 30 degrees. If the pt is found to have a large dural tear, surgery is
indicated
53. Diabetes Insipidus etiologies, clinical manifestations, diagnostic findings
Diabetes insipidus is a water metabolism problem caused by an ADH (antidiuretic hormone) deficiency (either a
decrease in ADH synthesis or an inability of the kidneys to respond to ADH)
· ADH deficiency results in the excretion of large volumes of dilute urine. w/o ADH, distal kidney tubules and
collecting ducts do not reabsorb water, leading to polyuria and dehydration
· *ensure that no patient suspected of DI is deprived of fluids for more than 4 hours, b/c he or she cannot reduce
urine output and severe dehydration can result
Etiology: Disorder of the posterior pituitary gland. DI is a water metabolism problem caused by an ADH (antidiuretic hormone) deficiency (either a decrease in ADH synthesis or an inability of the kidneys to respond to
ADH). ADH deficiency results in the excretion of large volumes of dilute urine. Without ADH, distal kidney tubules
and collecting ducts do not reabsorb water, leading to polyuria (excessive water loss through urination) and
dehydration.
o Dehydration caused by this massive water loss increases plasma osmolarity which increases sensation of
thirst which helps maintain water homeostasis. If thirst mechanism is poor or absent or if the pt is
unable to obtain water, dehydration becomes more severe and can lead to death.
o ADH deficiency is classified as nephrogenic, drug related, primary or secondary.
o Nephrogenic is inherited. Kidney tubules do not respond to the actions of ADH poor water
reabsorption by the kidneys. The amount of hormone produced is not deficient.
o Primary is caused by a defect in the hypothalamus or pituitary gland, resulting in lack of ADH production
or release. Secondary can result from tumors in or near the hypothalamus or pituitary, head trauma,
infectious process, surgical procedures.
o Drug-related is caused by lithium carbonate which can interfere with the response of the kidneys to
ADH.
Manifestations: most are related to dehydration (poor skin turgor, dry cracked mucus membranes)
o Key manifestations increased frequency of urination and excessive thirst
o Cardiovascular- hypotension, decreased pulse pressure, tachycardia, weak peripheral pulses, hemoconcentration (↑Hgb, ↑Hct, ↑BUN)
o Kidney/urinary- increased urine output (dilute, low spec grav, hypo-osmolar)
o Skin- poor turgor, dry mucous membranes
o Neuro- increased thirst sensation, irritability, decreased cognition, hyperthermia, lethargy to coma,
ataxia
23
Diagnostic: Water loss produces changes in blood and urine tests. The first step in diagnosis is to measure a 24-hr
fluid intake and output without restricting food or fluid intake
o Fluid output >4 L and > than volume ingested
o Amount of urine excreted in 24 hrs may vary from 4-30 L/day
Urine is dilute with Low spec grav <1.005 and low osmolarity 50-200 mOsm/kg
54. DKA etiologies, clinical manifestations, collaborative treatment
o DKA is characterized by uncontrolled hyperglycemia, metabolic acidosis, and increased production of ketones.
o Results from the combination of insulin deficiency and an increase in counter-regulatory hormone release
o Hormonal changes lead to increased liver and kidney glucose production and decreased glucose use in peripheral
tissues. Increased production of counter-regulatory hormones leads to the production of keto-acids, which results
in metabolic acidosis.
Etiologies:
o DKA occurs most often in pts w/type 1 DM but can also occur in those w/type 2 DM who are under severe stress
(like trauma, surgery, infection).
o The most common precipitating factor for development of DKA is infection. Death occurs in up to 10% of these
cases even w/appropriate tx.
o Mortality is highest for older pts who also have infection, stroke, MI, vascular thrombosis, intestinal obstruction, or
pneumonia
o Precipitating factors: infection, other stressors,
o inadequate insulin dose – profound insulin deficiency is the major cause
Clinical manifestations:
o Hyperglycemia leads to osmotic diuresis w/dehydration and electrolyte loss
o Classic symptoms: polyuria, polydipsia, polyphagia, weight loss, vomiting, abdominal pain, dehydration, weakness,
altered mental status, shock and coma.
o Ketosis: kussmaul respirations, “fruity” breath, nausea, abdominal pain
o Dehydration or electrolyte loss: polyuria, polydipsia, weight loss, dry skin, sunken eyes, soft eyeballs, lethargy,
coma; hypothermia, orthostatic hypotension, decreased neck veins, poor skin turgor, vomiting, diarrhea
o Hyperpnea, acetone breath, malaise; acute abdomen (absent bowel sounds, rebound tenderness)
o Mental status can vary from total alertness to profound coma –may be alert, obtunded, stuperous, hyporeflexia
(may be secondary to hypokalemia), hypotonia
o As ketone levels rise, the buffering capacity of the body is exceeded, the pH of the blood decreases and acidosis
occurs
o Kussmaul respirations (very deep and rapid respirations) cause respiratory alkalosis in an attempt to correct
metabolic acidosis by exhaling CO2
o Initial Na levels may be low or normal; initial K levels depend on how long DKA lasts before tx; after therapy starts,
K levels drop quickly
Collaborative treatment:
o Blood glucose management: assess airway, LOC, hydration status, electrolytes and BG levels; check pt’s BP and RR
q15 mins until stable; record urine output, temp, and mental status every hour; assess CVP q30 mins or as
prescribed if there is a central venous catheter present
o Fluid and electrolyte management:
- initial fluid bolus to restore volume and maintain perfusion to the brain, heart and kidneys
- hypotonic fluids like ½ NS or NS
- when BG levels reach 250, give 5% dextrose in ½ NS to prevent hypoglycemia and cerebral edema (which can
occur when serum osmolarity declines too rapidly)
o Drug therapy:
24
- insulin therapy: unless the episode of DKA is mild, regular insulin by continuous IV infusion is the tx of choice;
subcutaneous insulin started when pt can take oral fluids and ketosis has stopped –BS should fall at a rate of 75100 mg/dl/hr; **all patients w/DKA need insulin**
o Acidosis management: acidosis is corrected w/fluid replacement and insulin therapy K replacement is initiated
after serum levels fall below normal to prevent hypokalemia; bicarbonate is used only for severe acidosis
o EKG (determines K status), NG tube, bladder catheter
55. Education to prevent DKA
Prevention through education: evaluate patient knowledge, common misjudgments made by pt are omitting insulin
when unable to eat, failure to monitor BS, failure to test urine for ketones
o Ingesting at least 150 g of carbohydrate daily reduces the risk for starvation ketosis
o After consulting a PCP, urge pt to additional rapid-acting (Lispro) or short-acting (regular) insulin based on BG
levels
o Instruct the pt and family to consult the PCP when: BG > 250, ketonuria lasts for more than 24 hrs, the pt can’t
take food or fluids, illness lasts more than 1-2 days
o Instruct them to detect hyperglycemia by monitoring BG levels when the pt is ill; illness can result in
dehydration w/DKA, HHS, or both; pt should NOT omit insulin therapy during illness
Sick Day Rules:
o Notify your healthcare provider that you are ill
o Monitor your BG levels q4hrs –increase frequency of BG monitoring
o Test your urine for ketones when your BG level > 240
o Continue to take insulin or oral antidiabetic agents - Supplemental doses of rapid acting insulin (Lispro) or short
acting insulin (regular) may be required
o To prevent dehydration: drink 8-12 oz of sugar-free liquids every hr that you are awake; if your BG level is
below your target range, drink fluids that contain sugar (normally drink 3 L of fluid daily and increase the
amount when infection is present)
o Continue to eat meals at regular times
o If unable to tolerate solid foods b/c of nausea, consume more easily tolerated foods or liquids equal to the
carb content of your usual meal; when nausea is present, pt should take liquids containing both glucose and
electrolytes (like Gatorade)
o Call your PCP for any of these danger signs: persistent n/v, moderate or large ketones, BG elevation after 2
supplemental doses of insulin, high (101.5F) temp or increasing fever; fever for more than 24 hrs
o Treat s/s (like diarrhea, nausea, vomiting, fever) as directed by PCP
o Get plenty of rest
56. Hypoglycemia Unawareness
o Problem in long-standing type 1 DM; problem occurs most often in pts who have had type 1 DM for 30 yrs or
longer
o Pts no longer have the warning symptoms of impending hypoglycemia that should prompt them to take
preventive action
o Impaired insulin counterregulation: pt may be unable to recover from hypoglycemia; decline in both glucagon
and epinephrine greatly diminishes the counterregulatory response and increases the risk for severe
hypoglycemia (called hypoglycemia unawareness)
o Normal responses to hypoglycemia: sympathetic NS assists persons w/diabetes to become aware of
hypoglycemia; typical responses to hypoglycemia are sweating and tachycardia; decline in glucagon and
epinephrine greatly diminish counter-regulatory response; this increases the risk for severe hypoglycemia w/o
symptoms
57. Education for patients with Diabetic Peripheral Neuropathy
o Neuropathy of the feet and legs can be delayed by keeping BG levels as near normal as possible
o Urge smoking cessation to reduce the risk for vascular disease
o Feet should be evaluated closely at least annually –teach pts about preventive foot care and the need for
examination of the feet and legs at each visit to a HCP
25
o Explain problems caused by loss of protective sensation, the importance of monitoring the feet daily, proper care of
the feet (including nail and skin care) and how to select appropriate footwear; teach family members how to inspect
and care for the pt’s feet if the pt can’t
(chart 67.7)
o Protect feet and other body areas where sensation is reduced (do not walk around in bare feet or stocking feet;
always wear shoes w/a protective sole)
o Be sure shoes are long enough and wide enough to prevent creating sores or blisters; teach pt change shoes by
midday and again in the evening; teach pts to avoid tight stockings or those that have constricting bands
o Provide a long break-in period for new shoes; do not wear new shoes for longer than 2 hrs at a time –advise pts
w/neuropathy to break in new shoes slowly to reduce the risk for blisters
o Avoid pointed-toe shoes and shoes w/heels higher than 2 inches
o Inspect your feet daily (w/a mirror for open areas or redness)
o Avoid extremes of temperature; wear warm clothing in the winter, esp. over hands, feet, and ears
o Test water temperature w/a thermometer when washing dishes or bathing; use warm water rather than hot water
(less than 110F)
o Use potholders when cooking
o Use gloves when washing dishes or gardening
o Do not eat foods that are “steaming hot,” allow them to cool before placing them in your mouth
o Eat foods that are high in fibers (like fruit, whole grain cereals, vegetables)
o Drink 2-3L of fluids (nonalcoholic) daily unless your HCP has told you to restrict fluid intake
o Get up from a lying or sitting position slowly; if you feel dizzy, sit back down until the dizziness fades before standing
and then stand in place for a few seconds before walking or using the stairs
o Look at your feet and the floor or ground where you are walking to assess how the ground, floor, or step changes to
prevent tripping or falling
o Avoid using area rugs, esp. those that slide easily
o Use handrails when going up or down steps
o Chart 67-9: foot care instructions
58. Lactulose (Cephulac)
o Promotes the excretion of ammonia in stool (management of encephalopathy)
o Drug therapy in cirrhosis
o Nursing management of esophageal varices: to cause excretion of ammonia and cause diarrhea
o C: osmotic, laxative
o Indication: Treatment of chronic constipation; Adjunct in the management of portal-systemic (hepatic)
encephalopathy (PSE).
o Action: Increases water content and softens the stool; Lowers the pH of the colon, which inhibits the diffusion of
ammonia from the colon into the blood, thereby reducing blood ammonia levels.
o Therapeutic effect: Relief of constipation; Decreased blood ammonia levels with improved mental status in PSE.
o AE: belching, cramps, distention, flatulence, diarrhea, hyperglycemia (diabetic patients)
59. Esophageal varices
o Varices are tortuous, enlarged and swollen veins which result from portal HTN; these collateral vessels contain little
elastic tissue and are very fragile; Do not tolerate high pressure in system and distend and bleed easily
o Large varices are more likely to bleed;
o Common complication of cirrhosis; most life-threatening complication of cirrhosis; continuous vomiting of blood
o Bleeding esophageal varices represent a life-threatening medical emergency
o May have significant blood loss
o Hematemesis: vomiting blood
o Changes in LOC or loss of consciousness may precede any observed bleeding; variceal bleeding can occur
spontaneously w/no precipitating factors
o Any activity that increases abdominal pressure may increase the likelihood of a variceal bleed, including heavy lifting
or vigorous physical exercise; chest trauma or dry, hard food in the esophagus can cause bleeding
26
60. Vasopressin
o C: antidiuretic hormone; vasopressor
o Indication: central diabetes insipidus d/t deficient ADH
o Therapeutic Effect(s):Decreased urine output and increased urine osmolality in diabetes insipidus; Increased BP
o Drug therapy in cirrhosis to prevent or manage hemorrhage (p. 1302)
(p. 1379)
o Trade: Vasopressin
o Purpose/action: an exogenous form of ADH that serves as a replacement; binds to kidney receptors and enhances the
reabsorption of water, thus reducing urine output
Nursing interventions:
o For the hospitalized patient, monitor for signs of water intoxication, such as listlessness, drowsiness, confusion, HA,
anuria, and weight gain b/c vasopressin-induced water intoxication can also lead to seizures, coma, and death.
o Warn the pt to not drink more than 3 L of fluids daily while on this drug b/c drug promotes fluid retention and can
lead to fluid overload.
o Teach the pt to weigh himself daily and to notify HCP if 2 lbs or more is gained in 24 hrs b/c a rapid increase in weight
is an indicator of excessive fluid retention and may require a change in drug dosage.
o Tell the pt to notify the HCP if he experiences a persistent HA or acute confusion b/c these are manifestations of water
toxicity, which must be treated before seizure activity occurs.
61. Nursing interventions for severe ascites and peripheral edema
Nutrition therapy: low sodium diet diet –explain the purpose of the restriction and advise the pt and family to read the
sodium content labels on all food and beverages; no table salt; suggest alternative flavoring additives; vitamin
supplements may be added to IV fluids b/c the liver can’t store vitamins; 3,000 calorie, high CHO, protein (depends
on stage), low fat, low sodium diet for ascites
· Drug therapy: diuretic (to reduce fluid accumulation and to prevent cardiac and respiratory problems); weigh pt daily,
measure daily intake and output, measure abdominal girth, document peripheral edema, and assess electrolyte
levels; pt may have oral or IV potassium supplement; usually combination of furosemide (Lasix) and spironolactone
(Aldactone) as combination diuretic therapy for tx of ascites
-abdominal assessment: listen for bowel sounds and assess for abdominal wall rigidity (pts at risk for spontaneous
bacterial peritonitis); send a sample of ascitic fluid for a culture before drug therapy begins; quintolones given for
SBP or combination antibiotics if pt has allergies; massive ascites can be detected as a distended abdomen with
bulging flanks; umbilicus may protrude; dilated abdominal veins (caput medusa); ongoing measurement of
abdominal girth (done at the end of expiration)
· Paracentesis: (p.1300, chart 61-1) explain the procedure and answer pt questions; obtain VS, including weight; ask pt
to void before the procedure to prevent injury to the bladder; position the pt in bed w/the HOB elevated; monitor VS
per protocol or physician’s request; measure the drainage and record accurately; describe the collected fluid; label
and send the fluid for lab analysis, document in the patient record that specimens were sent; after the physician
removes the catheter, apply a dressing to the site, assess for leakage; maintain bedrest per protocol; weight the pt
after the paracentesis, document in the pt record both before and after paracentesis; needle puncture of abdomen;
peritoneoventous shunt (aka leven shunt) drains ascites through a one-way valve from the abdominal cavity to the
superior vena cava
· Respiratory support: for the pt w/hepatopulmonary syndrome, monitor oxygen saturation w/pulse oximetry, apply
O2 therapy if needed, elevate HOB to at least 30 degrees or as high as the pt wants to improve breathing and feet
elevated to decrease dependent ankle edema, often will relieve dyspnea, weight the pt daily (or delegate and
supervise this activity); auscultate lungs q 4-8 hrs for crackles that could indicate pulmonary complications,
depending on the pt’s overall condition
· Carefully monitor pt’s fluid and electrolyte status: specifically lab tests like BUN, serum protein, Hct, and electrolytes;
an elevated BUN, decreased serum proteins, and increased hematocrit may indicate hypovolemia
62. Planning outcomes for patients with acute pancreatitis
27
1. Pain relief = #1 intervention after protecting airway (they might be vomiting blood so might need NG tube):
administer pain medications, promote pancreatic rest (no eating to prevent release of enzymes); provide comfort
measures
2. Normal fluid and electrolytes
3. Minimal to absence of complications
4. Absence of recurrent attacks
·
Deficient fluid volume: monitor VS, hemodynamic monitoring, monitor ECG,lab values, I&O, assessment of edema,
adventitious lung sounds, skin turgor, mucous membranes, abdominal girth and urine output
·
Imbalanced nutrition: less than body requirements: hyperalimentation (TPN) and lipids; daily weights, tissue
integrity, and presence of adequate body fat and muscle mass assessments; replacement pancreatic enzymes
·
Ineffective breathing pattern: respiratory assessment, lung assessment for atelectasis, crackles, and rhonchi;
enhance full inspiration; positioning in semi-Fowlers
·
Ineffective therapeutic management: teach pt to abstain from alcohol, restrict fats and avoid rich and stimulating
foods, use more carbohydrates in diet, correctly measure blood glucose levels, observe for steatorrhea, assess patient’s
understanding of prescribed regimen, suggest f/u if alcohol use problematic
63. Collaborative management of the patient with acute pancreatitis
Focused on supportive care
·
1. Aggressive hydration to avoid shock: if shock present, blood volume replacements (dextran or albumin) can be
given (volume expanders); F&E imbalances are corrected w/LR; CVP readings can assist in fluid replacement
requirements; vasoactive drugs can be used to increase vascular resistance
·
2. Pain management w/Demerol (but can cause seizures) so use morphine (longer ½ life) and dilaudid;
antispasmotics/spasmolytic; avoid atropine-like drugs when paralytic ileus is present as they may contribute to the
problem; other drugs that relax smooth muscle like nitroglycerin and papaverine
·
3. Management of metabolic complications
·
4. Minimizing pancreatic stimulation (NPO) might not be able to absorb food
·
Nonsurgical management: fasting, blood volume replacement
·
Other collaborative therapy: NPO w/NGT to suction; iv calcium gluconate; H2-receptors antagonists or PPIs (to
decrease gastric secretions, protonix); antibiotics (to prevent peritonitis)
·
Surgical therapy: if pancreatic related gallstones, urgent ERCP is indicated; percutaneous drainage of pseudocysts
w/drainage tube left in place
Short Answer Prompts
1. Cerebral Perfusion Pressure (CPP)
The CPP is the pressure gradient over which the brain is perfused. It is influenced by oxygenation, cerebral blood
volume, blood pressure, cerebral edema. Maintenance of a CPP above 70 mm Hg is generally accepted as an expected
outcome of therapy.
2. Pathophysiologic process of increased intracranial pressure (ICP)
- Monroe-Kellie: The cranial contents include brain tissue, blood and CSF. These components are encased in the
relatively rigid skull. Within this space, there is little room for any of the components to expand or increase in
volume. The normal level of ICP is 10-15 mm Hg. Since the skull is a non-expandable, non-contractible, freely
communicating space, the pressure of the fluid contents and the brain itself must be directly proportional to
each other in order to maintain a constant pressure. If one of these pressures increases, another must decrease
to compensate.
- Any increase in volume of one component must be compensated for by the other components. As a first
response to an increase in volume of any of these components, the CSF is shunted or displaced from the cranial
compartment to the spinal subarachnoid space or rate of CSF absorption is increased. An additional response if
needed is a decrease in cerebral blood volume by displacement of the cerebral venous blood into the sinuses.
28
-
It occurs when compliance no longer takes place and the brain cannot accommodate further volume changes.
As ICP increases, cerebral perfusion decreases, leading to tissue hypoxia, a decrease in serum pH level, and an
increase in the level of carbon dioxide. This process causes cerebral vasodilation, edema, and a further increase
in ICP, and the cycle continues.
1) Increase in volume of either brain tissue, blood or CSF (↑ICP)
2) CSF shunted or displaced from cranial compartment to spinal subarachnoid space
3) Decrease in cerebral blood volume (↓Cerebral perfusion)
4) Tissue hypoxia
5) Decrease serum pH and increase CO2
6) Cerebral vasodilation
7) Edema
8) Increase ICP (cycle continues)
Two types of edema may cause increased ICP: vasogenic edema and cytotoxic edema.
Vasogenic edema is seen most often as a cause of increased ICP in the adult. It involves an increase in the
volume of brain tissue. This increase is caused by an abnormal permeability of the walls of the cerebral vessels,
which allows protein- rich plasma infiltrate to leak into the extracellular space of the brain. The fluid collects
primarily in the white matter.
Cytotoxic, or cellular edema may occur as result of a hypoxic insult, which causes a disturbance in cellular
metabolism, the sodium pump, and active ion transport. The brain is quickly depleted of available oxygen,
glucose, and glycogen and converts to anaerobic metabolism. The sodium pump fails, and sodium enters the
cells and pulls water from the extracellular space into the tissue. The serum sodium level decreases to less than
120 mEq/L (hyponatremia). As a result, an abnormal accumulation of fluid in the brain cells and decreases in the
extracellular fluid space occur. Cytotoxic edema may lead to vasogenic edema and further increase ICP.
Interstitial edema occurs in the presence of acute brain swelling and is associated with elevated BP or increased
CSF pressure. Edema develops rapidly in the perivascular and periventricular white space and can be controlled
through measures to reduce BP, decrease CSF pressures or increase cerebral perfusion pressure (CPP)
CPP: the pressure gradient over which the brain is perfused. It is influenced by oxygenation, cerebral edema, and
ICP and is determined by subtracting the mean ICP from the MAP (MAP-mean ICP). Maintenance of a CPP above
70 mmHg is goal.
1/3(SBP-DBP)+DBP = MAP
Example: Lets say a patient’s BP = 120/60.
Pulse pressure (SBP-DBP) would be 120-60 = 60.
Mean Arterial Formula: 1/3(SBP-DBP)+DBP = MAP
1/3 X 60 = 20
Add the result above to DBP (60)
29
20+60 = 80
3. Autonomic Dysreflexia (Hyperreflexia)
Autonomic Dyreflexia. Pathophysiology, etiologies, and collaborative interventions:
1. Occurs in injuries Above the sixth thoracic vertebra
2. Occurs after period of spinal shock
3. Results from uninhibited sympathetic discharge
Autonomic dysreflexia, also known as hyperreflexia, is a state that is unique to patients after spinal cord injury at
a T-5 level and above. Patients with spinal cord injuries at Thoracic 5 (T-5) level and above are very susceptible.
Autonomic dysreflexia can develop suddenly, and is a possible emergency situation. If not treated promptly and
correctly, it may lead to seizures, stroke, and even death.
Autonomic dysreflexia means an over-activity of the Autonomic Nervous System.
It can occur when an irritating stimulus is introduced to the body below the level of spinal cord injury, such as an
overfull bladder.
The stimulus sends nerve impulses to the spinal cord, where they travel upward until they are blocked by the
lesion at the level of injury. Since the impulses cannot reach the brain, a reflex is activated that increases activity
of the sympathetic portion of autonomic nervous system.
This results in spasms and a narrowing of the blood vessels, which causes a rise in the blood pressure. Nerve
receptors in the heart and blood vessels detect this rise in blood pressure and send a message to the brain.
The brain sends a message to the heart, causing the heartbeat to slow down and the blood vessels above the
level of injury to dilate.
However, the brain cannot send messages below the level of injury, due to the spinal cord lesion, and therefore
the blood pressure cannot be regulated.
-Clinical Manifestations
o Pounding headache (caused by the elevation in blood pressure)
o Goose Pimples/piloerection
o Sweating and flushing above the level of injury
o Pail extremetities below level of lesion
o Nasal stuffiness
o Bradycardia- Slow Pulse
o Blotching of the Skin
o Restlessness/ feeling of apprehension
o Nausea, blurred vision
- Most Common Causes
The most common cause seems to be overfilling of the bladder. This could be due to a blockage in the urinary
drainage device, bladder infection (cystitis), inadequate bladder emptying, bladder spasms, or possibly stones in
the bladder.
The second most common cause is a bowel that is full of stool or gas. Any stimulus to the rectum, such as digital
stimulation, can trigger a reaction, leading to autonomic dysreflexia.
Other causes include skin irritations, wounds, pressure sores, burns, broken bones, pregnancy, ingrown toenails,
appendicitis, and other medical complications.
Other causes: If your bladder has not triggered the episode of autonomic dysreflexia, the cause may be your
Bowel. Perform a digital stimulation and empty your bowel. If you are performing a digital stimulation when the
symptoms first appear, stop the procedure and resume after the symptoms subside.
If your bladder or bowel are not the cause, check to see if:
You have a pressure sore
30
You have an ingrown toenail
You have a fractured bone.
- Treatment
Treatment of autonomic dysreflexia must be initiated quickly to prevent complications.
Place patient in sitting position is priority! always keep head elevated.
Notify HCP
Loosen tight clothing
Assess for and treat the cause
Since a full bladder is the most common cause- check bladder for distention and catheterize immediately
Place anesthetic ointment on tip of catheter before instertion
If you have an indwelling catheter
check the urinary drainage system. If you have a Foley or suprapubic catheter, check the following:
¨Is your drainage full? Keep the drainage bags empty
¨Is there a kink in the tubing?
¨ Is the drainage bag at a higher level than your bladder?
Is the catheter plugged?
Check daily for grits (deposits) inside of the catheter.
If you are on an intermittent catheterization program, catheterize yourself as often as necessary to prevent
overfilling.
If you have spontaneous voiding, make sure you have an adequate output.
Carry spare clothes and an intermittent catheter kit when you are away from home.
Maintain a regular bowel program.
Perform routine skin assessments.Have a yearly re-evaluation.
Check patient for fecal impact; disimpact immediately using anesthetic ointment
Check Temp in room to make sure its not too cool or drafty
Monitor BP Q10-15 min
Give nitrates or hydralazine
Overview
Autonomic dysreflexia is a potentially dangerous clinical syndrome that develops in individuals with spinal cord
injury, resulting in acute, uncontrolled hypertension. All caregivers, practitioners, and therapists who interact
with individuals with spinal cord injuries must be aware of this syndrome, recognize the symptoms, and
understand the causes and treatment algorithm.
Briefly, autonomic dysreflexia develops in individuals with a neurologic level of spinal cord injury at or above the
sixth thoracic vertebral level (T6). Autonomic dysreflexia causes an imbalanced reflex sympathetic discharge,
leading to potentially life-threatening hypertension. It is considered a medical emergency and must be
recognized immediately. If left untreated, autonomic dysreflexia can cause seizures, retinal hemorrhage,
pulmonary edema, renal insufficiency, myocardial infarction, cerebral hemorrhage, and death. Complications
associated with autonomic dysreflexia result directly from sustained, severe peripheral hypertension. (See the
image below.)
31
(A) A strong sensory input (not necessarily noxious) is carried into the spinal cord via intact peripheral nerves.
The most common origins are bladder and bowel.
(B) This strong sensory input travels up the spinal cord and evokes a massive reflex sympathetic surge from the
thoracolumbar sympathetic nerves, causing widespread vasoconstriction, most significantly in the
subdiaphragmatic (or splanchnic) vasculature. Thus, peripheral arterial hypertension occurs.
(C) The brain detects this hypertensive crisis through intact baroreceptors in the neck delivered to the brain
through cranial nerves IX and X.
(D) The brain attempts two maneuvers to halt the progression of this hypertensive crisis. First, the brain
attempts to shut down the sympathetic surge by sending descending inhibitory impulses. These impulses are
unable to travel to most sympathetic outflow levels because of the spinal cord injury at T6 or above. Inhibitory
impulses are blocked in the injured spinal cord.
In the second maneuver, the brain attempts to bring down peripheral blood pressure by slowing the heart rate
through an intact vagus (parasympathetic) nerve; however, this compensatory bradycardia is inadequate and
hypertension continues. In summary, the sympathetics prevail below the level of neurologic injury, and the
parasympathetic nerves prevail above the level of injury. Once the inciting stimulus is removed, reflex
hypertension resolves.
4. Babinski reflex
- Babinski's reflex occurs when the big toe moves toward the
top surface of the foot and the other toes fan out after the sole of the foot has been firmly stroked.
- Babinski's reflex is one of the reflexes that occurs in infants. It
is normal in children up to 2 years old, but it disappears as the child gets older and the nervous system
becomes more developed. It may disappear as early as 12 months.
- The presence of a Babinski's reflex after age 2 is a sign of
damage to the nerve paths connecting the spinal cord and the brain (the corticospinal tract).
This tract runs down both sides of the spinal cord. A Babinski's reflex can occur on one side or on both
sides of the body.
- An abnormal Babinski's reflex can be temporary or
permanent.
o
o
o
o
Dorsiflexion of the great toe and fanning of the other toes is abnormal in anyone older than 2 yrs old and
represents presence of CNS disease.
Positive Babinski sign “upgoing toes” abnormal response
Negative Babinski “downgoing toes” normal
Babinski’s sign is a pathologic, or abnormal reflex.
32
4. Mechanisms of spinal cord injury
o
o
o
o
o
When sufficient force is applied to the spinal cord, damage results in neurologic deficits.
Sources of force include:
o Fracture
o Dislocation
o Subluxation
o Penetrating trauma [GSW, Knife Wounds]
o Although in some cases the cord itself may remain intact, at other times the cord undergoes a
destructive process caused by a contusion (bruise), compression, laceration and concussion.
Causes of SCI can be divided into primary and secondary mechanisms of injury.
4 primary mechanisms
o Hyperflexion- when the head is suddenly and forcefully accelerated forward, causing extreme flexion of
the neck.
*This type of injury often occurs in head-on collisions and diving accidents.
*Either process can disrupt the integrity of the spinal cord, causing hemorrhage, edema and necrosis.
o Hyperextension- Occur most often in MVA’s in which the patient’s vehicle is struck from behind or
during falls when the patient’s chin is struck.
*The head is suddenly accelerated and then decelerated. This stretches or tears the anterior longitudinal
ligament, fractures or subluxates the vertebrae.
o Axial Loading , or vertical compression
*Diving accidents, falls on the buttocks, or a jump in which the person lands on the feet can cause many of
the injuries attributed to axial loading. The blow to the top of the head causes the vertebrae to shatter.
*Pieces of bone enter the spinal canal and damage the cord.
o Excessive rotation- caused by turning the head beyond normal range. Penetrating injuries - classified by
the speed of the object (knife, bullet). GSW cause direct and indirect damage.
Secondary injuries exacerbates the primary injury and worsens morbidity.
Include:
o
o
o
Neurogenic shock- a type of hypovolemic shock (especially in pts with cervical spine injuries
Vascular insult
Hemorrhage- and loss of blood vessel tone (dilation) after severe cord injury may result in
hypovolemia
o
Ischemia
33
o
o
o
Fluid and Electrolyte imbalance
Spinal cord edema occurs as a result of cord compression by hemorrhage, bony fragments or lacerations.
Necrosis of the spinal cord occurs from compromised capillary circulation and venous return.
4. Diabetic Ketoacidosis (DKA) vs Hyperosmotic Hyperglycemic Nonketotic Syndrome (HHNS)
Diabetic ketoacidosis
o Severe insulin deficiency causes hydrolysis of triglycerides; yielding glycerol, lactate and free fatty acids
(FFA’s)
o Increased glycerol and FFA’s result in lypolysis (Breakdown of fats)
o Excess FFA’s cause excessive amounts of ketone bodies to form in liver
o FFA’s converted to ketone bodies
o Ketone bodies (weak acids) can be used by most body tissues
CHARACTERISTICS INCLUDE:
1. Hyperglycemia
2. Ketosis
3. Dehydration
4. Severe Acidosis
5. Variable changes in level of consciousness [LOC]
PRECIPITATING FACTORS
ILLNESS AND INFECTION (25 –30%) OF CASES
SNS STIMULATION CAUSING INCREASE IN EPINEPHRINE AND NOREPINEPHRINE LEVELS CAUSING
GLYCOGENOLYSIS INADEQUATE INSULIN MAJOR FACTOR
WHY DOES IT OCCUR?
Profound Insulin Deficiency is the major cause
Insulin deficiency impairs glucose uptake
Continuing fasting results in excess glucose production
Hyperglycemia causes impaired metabolism of carbohydrates, fats and proteins
MANIFESTATIONS
RESPIRATORY SYMPTOMS
VERY DEEP, RAPID BREATHING (KUSSMAUL RESPIRATIONS)
HYPERVENTILATION IS BODY’S ATTEMPT TO PRODUCE RESPIRATORY ALKALOSIS BY BLOWING OFF PRODUCTS
OF ACIDEMIA
HYPOTHERMIA
HYPERPNEA (DEEP RESPIRATIONS)
ACETONE BREATH (ACETOACETATE, A KETONE BODY IS CONVERTED TO ACETONE AND EXCRETED BY THE
LUNGS (FRUITY ODOR)
DEHYDRATION (INTRAVASCULAR DEPLETION (COMMON SIGN)
3 “P’S”
POLYURIA
POLYDYPSIA
POLYPHAGIA
WEIGHT LOSS
NONSPECIFIC SYMPTOMS (MALAISE)
G.I. SYMPTOMS (N/V, ABD PAIN)
34
HYPERGLYCEMIC HYPEROSMOLAR NONKETOTIC SYNDROME (HHNS)
o SOMETIMES OVERLOOKED AND OFTEN CONFUSED WITH OTHER ILLNESSES
o OFTEN OCCURS IN ELDERLY WITH EITHER TYPE 1 OR TYPE 2 DM
o HHNS OCCURS IN ELDERLY PATIENTS WHOSE DIABETES IS TREATED WITH MEDICAL NUTRITION THERAPY
(MNT) WITH OR WITHOUT ORAL ANTIDIABETIC AGENTS AND WHO MAY BE INADEQUATELY MONITORED
o S/S MAY GO UNRECOGNIZED FOR WEEKS
PATHO
SYNDROME WITH 4 PRIMARY FEATURES:
SEVERE HYPERCLYCEMIA
ABSENCE OF KETOSIS
PROFOUND DEHYDRATION
NEUROLOGIC MANIFESTATIONS
HHNS SIMILAR TO DKA EXCEPT THAT INSULIN DEFICIENCY IS PROBABLY LESS PROFOUND
LYPOLYSIS DOES NOT OCCUR (ABSENCE OF KETOSIS DECREASES G.I. SYMPTOMS, SO PATIENT DOES NOT SEEK
MEDICAL HELP)
IMPAIRED THIRST MECHANISMS
INDIVIDUAL INABILITY TO REPLACE FLUIDS ESPECIALLY IN THE ELDERLY
BECAUSE HHNS PATIENTS ARE DEHYDRATED, MENTATION CHANGES ARE MORE COMMONLY SEEN THAN IN DKA
SEVERE DEHYDRATION LEADS TO MORE FREQUENT AND EARLY CHANGES IN LEVEL OF CONSCIOUNESS
PRECIPITATING FACTORS
MASSIVE FLUID LOSS FROM PROLONGED OSMOTIC DIURESIS SECONDARY TO HYPERGLYCEMIA
SEVERE BURNS, DIARRHEA
HEMO OR PERITONEAL DIALYSIS
HYPERTONIC FEEDING (PROLONGED TPN, HIGH PROTEIN NGT FEEDINGS
MYOCARDIAL INFARCTION
G.I. HEMORRHAGE
PHARMOCOLOGIC AGENTS (THIAZIDES, PROPANOLOL, PHENYTOIN, STEROIDS, FUROSEMIDE)
SIGNS AND SYMPTOMS
S/S OF HHNS SIMILAR TO DKA WITH “SEVERAL IMPORTANT EXCEPTIONS”
G.I. SYMPTOMS MILDER
KUSSMAUL RESPIRATIONS SELDOM OBSERVED (LACK OF ACIDOSIS)
FOCAL NEUROLOGICAL SIGNS (MAY MIMIC CVA)
LABS: KETONE BODIES ARE NOT PRESENT EXCEPT FOR VERY SMALL AMOUNTS, OSMOLARITY IS MARKEDLY
ELEVATED, GLUCOSE USUALLY > 800
TREATMENT
PRIMARY TREATMENT GOAL IS “REHYDRATION”
RESTORE CIRCULATING PLASMA VOLUME
CORRECT ELECTROLYTE DEFICITS
ADEQUATE INSULIN
PREVENT COMPLICATIONS
RX UNDERLYING MEDICAL CONDITIONS
PROVIDE PATIENT & FAMILY EDUCATION
REHYDRATION: FLUID REPLACEMENT DEPENDS ON PATIENT’S STATE OF HYDRATION AND CARDIOVASCULAR
STATUS*** USE CAUTION WHEN REHYDRATING THE ELDERLY PATIENT TO AVOID FLUID OVERLOAD ***
PREVENTION: TO PREVENT HHNS FROM REOCCURRING:
IDENTIFY HIGH RISK PATIENTS
35
ENCOURAGE ADEQUATE HYDRATION
EDUCATE ELDERLY CLIENT ABOUT SICK-DAY CARE
COMPARISON WITH DKA
THE ABSENCE OF “SIGNIFICANT” KETOSIS DIFFERENTIATES HHNS FROM DKA
MORTALITY IS HIGHER IN HHNS THAN DKA SECONDARY TO “SEVERE” METABOLIC CHANGES, DELAY IN
DIAGNOSIS AND OTHER MEDICAL COMPLICATIONS
36