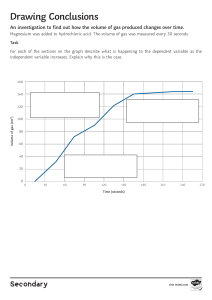
Class Adm No Candidate Name: 2021 Weighted Assessment 2 Pre-University 1 H2 CHEMISTRY 9729 Multiple-Choice & Structured Questions 28 July 2021 1 hour 15 minutes Candidates answer on the Question paper. Additional materials: Data Booklet READ THESE INSTRUCTIONS FIRST Do not turn over this question paper until you are told to do so. Write your name, class on all the work you hand in. Write in dark blue or black pen. You may use an HB pencil for any diagrams or graphs. Do not use staples, paper clips, glue or correction fluid. Answer all questions. All numerical answers should be given to 3 significant figures, and units must be given where appropriate. The use of an approved scientific calculator is expected, where appropriate. A Data Booklet is provided. At the end of the examination, fasten all your work securely together. The number of marks is given in brackets [ ] at the end of each question or part question. Question MCQ 10 11 12 13 Total Marks 9 7 8 9 7 This question paper consists of 8 printed pages and 2 blank pages. 40 2 Section A – Multiple Choice For each question there are four possible answers, A, B, C, and D. Choose the one you consider to be correct and fill in your answers to Section A in the boxes below. 1 Q1 Q2 Q3 Q4 Q6 Q7 Q8 Q9 Q5 When passed through an electric field, the 1H+ ion is deflected as shown below. A 1 H+ − B o 4 o 4 C source D + Which of the above beams represents the deflection for an ion 2X2– when passed through the same electric field? 2 Use of the Data Booklet is relevant to this question. Which of the following species has the same electronic configuration as Mn+? 3 A Cr B Cu C Fe2+ D V+ In which of the following pairs is the bond angle in the first species smaller than that in the second species? 1 PBr3, PBr4+ 2 H2Se, H2O 3 SF2, SCl2 A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3 3 4 The successive ionisation energies, in kJ mol–1, of elements G and H are given below. G H 580 940 1820 2050 2740 2970 11600 4140 14800 6590 18400 7880 23300 14900 Which statements about elements G and H are true? 1 The first ionisation energy of G is lower than that of the element preceding it in the Periodic Table. 5 2 G and H forms a compound with the formula G3H2. 3 H has an outer electronic configuration ns2 np4. A 1 and 2 only B C 1 and 3 only 2 and 3 only D 1, 2 and 3 Propanone is known commonly as acetone and is used as a nail polish remover. How many σ and π bonds are there in the molecule? 6 σ bonds π bonds A 8 1 B 8 2 C 9 1 D 9 2 40 cm3 of an unknown hydrocarbon, CxHy, was exploded in 280 cm3 oxygen. The volume of the residual gases was found to be 200 cm3. On being shaken with potassium hydroxide, the final volume was found to be 80 cm3. What is the molecular formula of the unknown hydrocarbon? (All volumes are measured at r.t.p) A C2H4 B C2H6 C C3H6 D C3H8 [Turn over 4 7 Use of the Data Booklet is relevant to this question. An element M can exist in a few oxidation states. 15.00 cm3 of an aqueous solution of 0.100 mol dm3 of Mn+ required 20.00 cm3 of 0.0250 mol dm3 of acidified K2Cr2O7 solution for a complete reaction. What is the change in oxidation state of M? A 8 +2 B C +3 D -2 -3 The bond lengths and bond angles in the molecules of methane, ammonia and water may be represented as follow: ammonia methane C 109.5° 0.109 nm water 0.101 nm N O 0.096 nm 105° 107° What causes this trend in the bond angles shown? 1 Increasing repulsion between hydrogen atoms as the bond length decreases. 2 Number of non–bonding electron pairs of the central atom in the molecule. 3 Non-bonding electron pair–bonding electron pair repulsion is greater than bonding electron pair–bonding electron pair repulsion. A 3 only B 1 and 2 only C 2 and 3 only D 1, 2 and 3 5 9 Trichloroisocyanuric acid is commonly used in swimming pools as a disinfectant. The recommended concentration level of the acid is 1.50 mg per dm3 of water. trichloroisocyanuric acid Mr = 232.5 How many chlorine atoms are present in a 2.50 x 106 dm3 Olympic–sized swimming pool filled with the recommended concentration level of trichloroisocyanuric acid? A 2.91 x 1025 B 2.91 x 1028 C 9.71 x 1024 D 9.71 x 1027 [Turn over 6 Section B: Structured Questions Answer all questions. 10 The graph below shows the first ionisation energy of the elements beryllium to magnesium. First ionisation energy / kJ mol-1 3000 2000 1000 0 Be Be (a) Be B C C N N O O F F Ne Ne Na Na Mg Mg Define, with the aid of an equation, the term first ionisation energy of sodium. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [2] (b) Account for the general increasing trend of ionisation energy from beryllium to neon. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [2] For Examiners’ Use 7 (c) Explain why the first ionisation energy decreases from beryllium to boron and from nitrogen to oxygen. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [2] (d) Explain why the first ionisation energy decreases sharply from neon to sodium. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [1] [Total: 7] [Turn over For Examiners’ Use 8 11 Group 2 metals are commonly known as alkali earth metals as they generally dissolve in water to form alkaline solutions. Magnesium and calcium are both group 2 metals. (a) Complete the table below for two isotopic species of magnesium. isotopic species [2] number of neutrons number of electrons 14 12 24 Mg2+ (b) State the structure and bonding in magnesium. Draw a labelled diagram to illustrate the structure of magnesium. ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [3] (c) Predict how the melting point of magnesium will differ from beryllium. Explain. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [3] [Total: 8] For Examiners’ Use 9 12 Magnesium oxide has a melting point of 2852 C. (a) For Examiners’ Use Explain, in terms of structure and bonding, why magnesium oxide has a higher melting point than sodium oxide, which has a melting point of 1950 °C. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [3] (b) Draw the ‘dot-and-cross’ diagrams for magnesium oxide, and for water. [2] (c) Magnesium ions in the aqueous state exists as the hydrate magnesium ion [Mg(H2O)6]2+, where a central magnesium ion is bonded to six water molecules via dative covalent bonds. (i) By considering the number of bond pairs and lone pairs of electrons around the central magnesium ion, deduce the shape and bond angle about the central magnesium ion in [Mg(H2O)6]2+. Shape: ……………………………………… Bond Angle: …………………………… [2] [Turn over 10 (ii) Explain why the magnesium ion is able to form dative covalent bonds with water molecules. …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… ……………………………………………………………………………………………….. [2] [Total: 9] For Examiners’ Use 11 13 To facilitate better plant growth, there is widespread use of fertilisers and soil additives in the agricultural sector. Agricultural lime is a soil additive used to increase soil pH so as to facilitate uptake of plant nutrients such as nitrogen and phosphorous. It is usually made up of a combination of calcium carbonate and magnesium carbonate. A manufacturer claims that a 2.00 g sample of agricultural lime contains 92 % calcium carbonate (Mr = 100.1) and 8 % magnesium carbonate (Mr = 84.3) by mass. To verify the manufacturer’s claim, the following steps were carried out: Step 1 The solid carbonate mixture was dissolved in 250 cm3 of 0.25 mol dm–3 hydrochloric acid. Step 2 A 25.0 cm3 portion of this resultant solution was titrated with 0.14 mol dm–3 potassium hydroxide. It was found that 12.50 cm3 of potassium hydroxide was required for complete neutralisation. (a) (i) Use the information above to calculate the amount of HCl in the 25.0 cm3 portion that reacted with potassium hydroxide in step 2. [1] (ii) Calculate the amount of excess HCl in the 250 cm3 volumetric flask in step 1. [1] [Turn over For Examiners’ Use 12 (iii) Calculate the amount of HCl that has reacted with the solid carbonate in step 1. [1] (iv) Hence calculate the total amount of carbonate present in the sample. [1] (b) Based on the manufacturer’s claim of 92% calcium carbonate and 8% magnesium carbonate by mass, calculate the amount of each carbonate present in the sample of agricultural lime. [2] 13 The manufacturer’s claim is considered valid if the difference between the actual and theoretical total amount of carbonate present in the sample is less than 0.0010 mol. (c) Using your answers in (a)(iv) and (b), determine if the manufacturer’s claim is valid. [1] [Total: 7] End of Weighted Assessment \ [Turn over 14 Blank Page