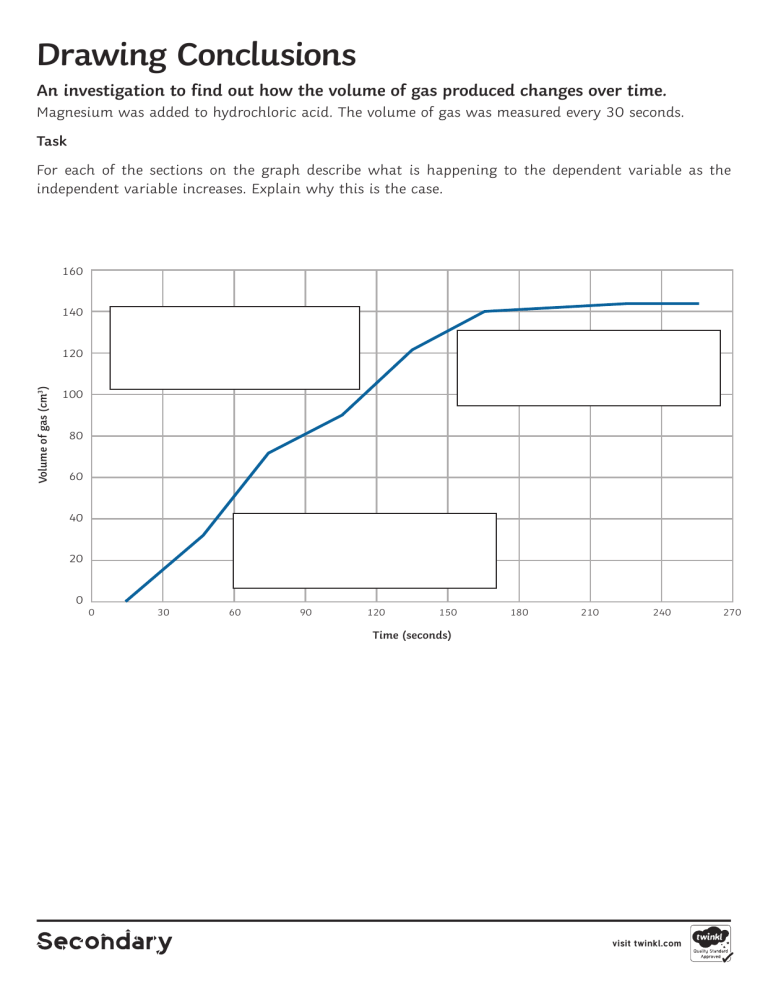
Drawing Conclusions An investigation to find out how the volume of gas produced changes over time. Magnesium was added to hydrochloric acid. The volume of gas was measured every 30 seconds. Task For each of the sections on the graph describe what is happening to the dependent variable as the independent variable increases. Explain why this is the case. 160 140 Volume of gas (cm3) 120 100 80 60 40 20 0 0 30 60 90 120 150 180 210 240 Time (seconds) visit twinkl.com 270 Drawing Conclusions Answers 160 140 2. As time increases, the volume of gas goes up steadily. Volume of gas (cm3) 120 3. As time increases, the volume of gas starts to plateau. 100 80 60 40 1. As time increases, the volume of gas goes up rapidly. 20 0 0 30 60 90 120 150 180 210 240 270 Time (seconds) Explain The volume of gas initially increases rapidly because there is a lot of magnesium and acid to react. The volume of gas being produced after about 60 seconds rises less rapidly because there is less magnesium and acid to react together, The volume of gas starts to plateau because either the magnesium has all reacted and/or the acid has all reacted. visit twinkl.com







