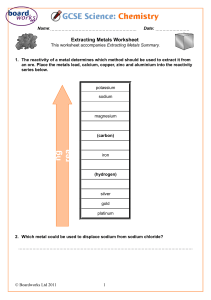
EXTRACTION OF IRON ESSENTIAL TERMS • METTALLURGY: BRANCH OF MATERIAL SCIENCE CONCERNED WITH THE EXTRACTION AND UTILIZATION OF METALS. • MINERALS: NATURALLY OCCURRING, HOMOGENOUS, CRYSTALLINE INORGANIC SOLID WITH A DEFINED COMPOSITION. • ORE: A MINERAL FROM WHICH A METAL CAN BE EXTRACTED PROFITABLY. • ALL ORES ARE MINERALS, BUT ALL MINERALS ARE NOT ORES. • GANGUE: PORTION OF THE ORE WHICH DOES NOT HAVE ANY COMMERCIAL VALUE • PROFITABLY MEANS THE COST OF GETTING THE METAL OUT OF THE ORE IS SUFFICIENTLY LESS THAN THE AMOUNT OF MONEY MADE BY SELLING THE METAL GENERAL STEPS Mining Pretreating magnetic attraction, cyclone separation, flotation, leaching Converting mineral to compound Converting compound to metal Refining Alloying Iron is very reactive and is generally found in nature in the forms of oxide, carbonates and sulfates. The main ores are: OCCURRENCE OF IRON Hematite (Fe2O3) Magnetite (Fe3O4) Siderite (FeCO3) Pyrite(FeS2) EXTRACTION OF IRON • IRON IS BELOW THE CARBON IN REACTIVITY SERIES IRON IN THE ORE WILL REDUCE TO THE IRON METAL BY HEATING WITH COKE OR CARBON • ORE TO IRON METAL INVOLVES A SERIES OF OVERALL REDOX +ACID BASE REACTIONS EVEN THOUGH THE DETAILED CHEMISTRY IS NOT UNDERSTOOD EVEN TODAY • THERE ARE 3 STEPS • 1. CONCENTRATION • 2. CALCINATION • 3. SMELTING 1. CONCENTRATION • ORE IS BROKEN INTO SMALL PIECES, AND IT IS SEPARATED BY A GRAVITY SEPARATION PROCESS IN WHICH IT IS WASHED WITH WATER TO REMOVE SAND AND CLAY ETC. 2. CALCINATION AND ROASTING • THE ORE IS HEATED IN THE ABSENCE OF AIR (CALCINATION) • AS A RESULT, CARBONATES DECOMPOSE INTO OXIDES • FERROUS OXIDE IS CONVERTED INTO FERRIC OXIDE 3. SMELTING • THE CONCENTRATED ORE IS MIXED WITH THE CALCULATED QUANTITY OF COKE AND LIMESTONE AND THE MIXTURE IS PUT IN THE BLAST FURNACE FROM THE TOP. WHAT IS HAPPENING IN THE BLAST FURNACE REACTIONS • HEIGHT 40 M • WIDTH 14 M RELATE THE METHOD OF EXTRACTION OF A METAL FROM ITS ORE TO ITS POSITION IN THE REACTIVITY SERIES. • The way we extract a metal from its ore depends on the position of the metal in the reactivity series. • Carbon is used to reduce oxides of metals below it in the reactivity series. • Metals above carbon in the reactivity series cannot be extracted from their oxides by heating with carbon. • This is because the metal bonds to oxygen too strongly and the carbon is not reactive enough to remove it. • So, we must use electrolysis to extract metals more reactive than carbon, such as aluminum, magnesium, and calcium. LINKS FOR MCQS • HTTPS://WWW.BBC.CO.UK/BITESIZE/GUIDES/ZV7F3K7/TEST • HTTPS://QUIZIZZ.COM/ADMIN/QUIZ/5E6EFE063A5DB3001C5D5612/EXTRACTION-OFIRON?SOURCE=MAINHEADER&PAGE=QUIZPAGE&SEARCHLOCALE= • HTTPS://PLAYER.QUIZALIZE.COM/QUIZ/695D99D1-FF94-4BD3-8A88-23529BBCFA69