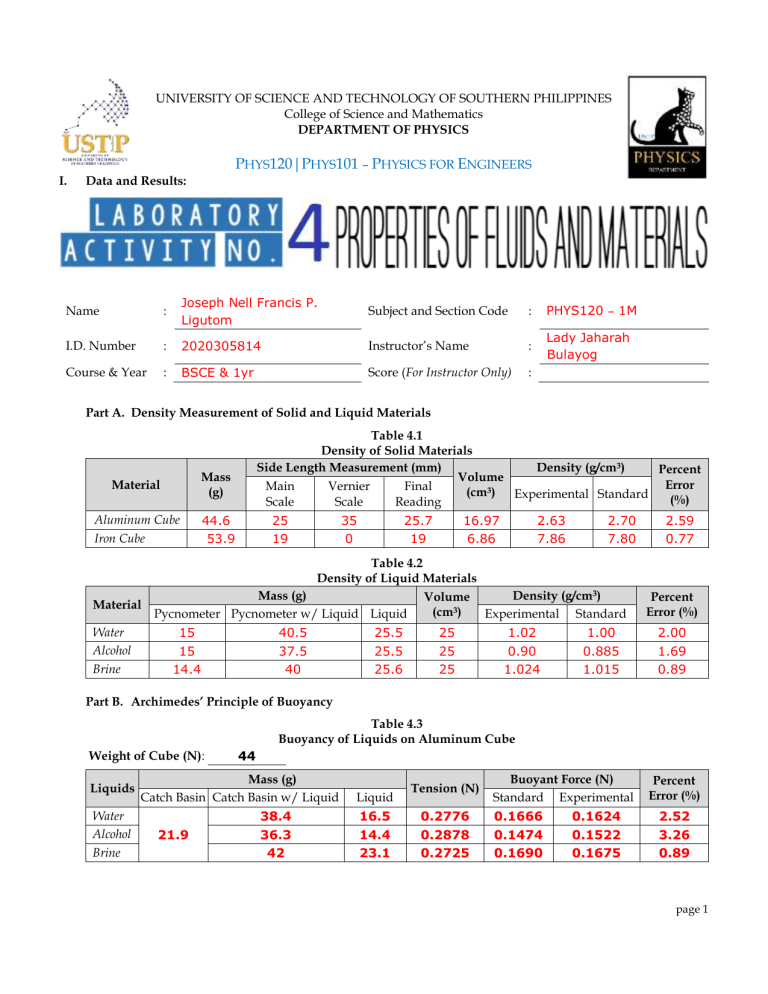
UNIVERSITY OF SCIENCE AND TECHNOLOGY OF SOUTHERN PHILIPPINES College of Science and Mathematics DEPARTMENT OF PHYSICS PHYS120|PHYS101 – PHYSICS FOR ENGINEERS I. Data and Results: Name : Joseph Nell Francis P. Ligutom Subject and Section Code : PHYS120 – 1M I.D. Number : 2020305814 Instructor’s Name : Lady Jaharah Bulayog Course & Year : BSCE & 1yr Score (For Instructor Only) : Part A. Density Measurement of Solid and Liquid Materials Material Mass (g) Aluminum Cube Iron Cube 44.6 53.9 Table 4.1 Density of Solid Materials Side Length Measurement (mm) Density (g/cm3) Percent Volume Error Main Vernier Final (cm3) Experimental Standard (%) Scale Scale Reading 25 19 35 0 25.7 19 16.97 6.86 2.63 7.86 2.70 7.80 2.59 0.77 Table 4.2 Density of Liquid Materials Material Water Alcohol Brine Mass (g) Pycnometer Pycnometer w/ Liquid Liquid 15 15 14.4 40.5 37.5 40 25.5 25.5 25.6 Volume (cm3) 25 25 25 Density (g/cm3) Experimental Standard 1.02 0.90 1.024 1.00 0.885 1.015 Percent Error (%) 2.00 1.69 0.89 Part B. Archimedes’ Principle of Buoyancy Table 4.3 Buoyancy of Liquids on Aluminum Cube Weight of Cube (N): Liquids Water Alcohol Brine 44 Mass (g) Catch Basin Catch Basin w/ Liquid 21.9 38.4 36.3 42 Liquid 16.5 14.4 23.1 Tension (N) 0.2776 0.2878 0.2725 Buoyant Force (N) Standard Experimental 0.1666 0.1474 0.1690 0.1624 0.1522 0.1675 Percent Error (%) 2.52 3.26 0.89 page 1 Table 4.4 Buoyancy of Liquids on Iron Cube Weight of Cube (N): Liquids Water Alcohol Brine II. 53 Mass (g) Catch Basin Catch Basin w/ Liquid 22.1 28.3 28.2 30.6 Liquid 6.2 6.1 8.5 Tension (N) 0.4674 0.4674 0.4571 Buoyant Force (N) Standard Experimental 0.0672 0.0595 0.0682 0.0626 0.0626 0.0729 Percent Error (%) 6.85 5.21 6.89 Computations: page 2 page 3 page 4 page 5 III. Discussion of Results: In this lab experiment we attained the objectives by getting the density of a liquid and solid material, determine the buoyant force of different materials and determine the effect of density to the buoyancy of a material. page 6 First in Table 5.1, we were able to identify the density of the solid materials such as the aluminum and iron cube. The masses were given the procedure, afterwards, we were able to read their length measurements in mm by reading through a Vernier caliper scale. The final readings could be computed by using the formula: main scale + (Vernier scale × 0.02mm). Volume in each solid were computed by converting the final readings to cm3 simply by multiplying it to 0.1 and then raised the value to its 3rd exponent. The density is computed by ; next is to compare it to its standard density and lastly determine its percentage error. In table 5.2, it discussed about the density of liquid materials of the following: water, alcohol and brine. Masses were computed as well as the volume, density and percentage error. The following results still can be seen in the said table. For part B, in tables 5.3 and 5.4, shows the buoyancy of liquids depending to the volume of the following solids: Aluminum and Iron. Same process have been used by computing different factors: mass, tension, buoyant force and percentage error. The difference in here is that the standard buoyant force has more specific formula as shown in the computations. Nevertheless, it tells us according to the results that buoyant force using the aluminum cube’s volume is greater compared to iron. IV. Conclusion: In this experiment, I can conclude that there is an existing relationship between the weight of water displaced by an object and the buoyant force exerted on the object. It can also be imply that the density of an object or fluid is a factor to determine whether a certain object will either float or sink when submerged by a liquid. The activity shows that buoyant force is page 7 greater for heavier objects; if the object is denser than the fluid, it can entail a downward acceleration which means to sink. In opposite, if the object is less dense, it will float or rise. V. Answers to Questions: 1. Consider the solid and liquid materials used in the activity. If each material has equal volume of 1 cm 3, which material weighs the heaviest? the lightest? Arrange the materials in order of increasing mass. How does their densities affect their masses for a given equal volume? The density is directly proportional with their masses for a given equal volume; greater density= heavier mass of material. So from the activity, the order of materials from lowest to heaviest are the following: Alcohol (0.885g), water (1.00 g), Brine (1.015g), Aluminum (2.70 g), lastly the heaviest is Iron (7.80g). 2. Consider the solid and liquid materials used in the activity. If each material has equal mass of 1 kg, which material occupies more space? less space? Arrange the materials in order of decreasing volume. How does their densities affect their volumes for a given equal mass? The density is inversely proportional with their volume for a given mass; the greater the density, the volume of the material will be lesser. This is according to the decreasing order of volume of the materials used in the activity. page 8 3. Which liquid exerts more buoyant force on each cube? Does the density of liquids affect the buoyant force exerted on the cube? Explain. It is Brine that exerts more buoyant force on each cube. It has the greatest density which means that density is directly proportional to the buoyant force that the liquid exerts on cube. Since it has greater density, means the buoyant force exerted on the cube is also greater. 4. What is the relationship between the weight of the collected liquid to the weight of the object in air? In this case, the buoyant force on a body floating in a liquid or gas is also equivalent in magnitude to the weight of the floating object and is opposite in direction; means that the object neither rises or sinks. Hence, weight of displaced portion of the fluid is equivalent to the magnitude of the buoyant force. page 9 5. Why is it easier to piggyback someone when you’re in swimming pool or sea as compared when you do it with just the surrounding air? This is because things appear lighter when you piggyback someone in the water because the fluid is actually helping to push it up. This is also in relation to Archimedes’ principle stating that force pushing on an object underwater is equal to the mass of the water it has pushed out of the way. In addition, since air is less dense than water, the buoyant force of air is less than that of water, means that object will be heavier. Thus, it will be easier to piggyback someone in the water than in the surrounding air. 6. Would it be easier to swim it sea than in river? Explain why. No; due to high content of salt in the sea water, the density of the sea water is higher compared to river water. From the data above, we can support our answer because the density is directly proportional to buoyant force. Therefore, since sea water is denser that the river water, the buoyant force is higher in the sea water compared to the river. Making it easier to swim in river water than sea water. Thus, it will not be easier to swim in the sea compared in the river. 7. For an object that is just dropped on a liquid, how does both the density of the object and liquid determine whether the object floats, sinks or just submerged under the surface of the liquid? If the density of the object is greater than the liquid, then the object sinks in the liquid. Otherwise, if the object is less dense than the liquid, then the object floats on the liquid. page 10 8. Does a floating object sink more in a liquid with low density than in a liquid with high density? Explain your answer. Depending on the weight of the floating object; If the liquid is denser, thus the buoyant force is greater than the object’s weight, means it will float. Otherwise if the liquid is less dense, thus the buoyant force is less than the mass of the object, in this case, the object will most likely to sink. Thus, the floating object would sink more in a liquid with less density than with the high density liquid. 9. From your data on the mass of the collected liquid in Table 5.3 and 5.4, will the volume of collected liquids match the volume of the cubes as recorded in Table 5.1? Show some calculations to prove or disprove. The volumes might not be close enough match; in table 5.3 for the aluminum cube with a volume of16.97��3, the masses of the corresponding displaced liquids are: 16.5 g (water), 14.4 g (alcohol), and 20.1 g (Brine). Converting mass into volume we just easily change the grams to cubic centimeters since1� = 1��3. In this sense, the volume of the displaced liquid will be close to the volume of the aluminum cube. Another proof is that in table 5.4 for the Iron cube with a volume of6.86��3, the masses of the corresponding displaced liquids are 6.2 g (water), 6.1 g (alcohol) and 8.5 (brine). Converting mass into volume we just simply change the grams to cubic centimeters again, since1� = 1��3. Thus, the volume of the displaced liquid is close to the volume of the iron cube. page 11 page 12