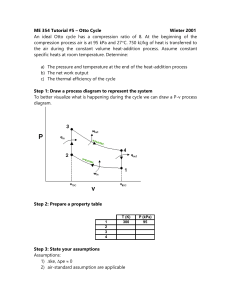
Thermodynamic Cycles • Look at different cycles that approximate real processes • You can categorize these processes several different ways • • • • Power Cycles vs. Refrigeration Gas vs. Vapor Closed vs. open Internal Combustion vs. External Combustion Power Cycles • Otto Cycle • Spark Ignition • Diesel Cycle • Brayton Cycle • Gas Turbine • Rankine Cycle These are all heat engines. They convert heat to work, so the efficiency is: Wnet η th = Qin Ideal Cycles • We’ll be using ideal cycles to analyze real systems, so lets start with the only ideal cycle we’ve studied so far Carnot Cycle Q W Q-W=0 Æ Q=W In addition, we know that the efficiency for a Carnot Cycle is: η th , Carnot TL = 1− TH Carnot Cycle is not a good model for most real processes • For example • Internal combustion engine • Gas turbine • We need to develop a new model, that is still ideal Air-Standard Assumptions • Air continuously circulates in a closed loop and behaves as an ideal gas • All the processes are internally reversible • Combustion is replaced by a heataddition process from the outside • Heat rejection replaces the exhaust process • Also assume a constant value for Cp, evaluated at room temperature Terminology for Reciprocating Devices Compression Ratio V max VBDC r= = V min VTDC Mean Effective Pressure 2 W = ∫ PdV 1 W = P∆V v v 1-2 2-3 3-4 4-1 Isentropic Compression Constant Volume Heat Addition Isentropic Expansion Constant Volume Heat Rejection Thermal Efficiency of the Otto Cycle Wnet Qnet Qin − Qout Qout η th = = 1− = = Qin Qin Qin Qin Apply First Law Closed System to Process 2-3, V = Constant Qnet , 23 − Wnet , 23 = ∆U 23 3 Wnet , 23 = Wother , 23 + Wb , 23 = 0 + ∫ PdV = 0 2 Qnet , 23 = ∆U 23 Qnet , 23 = Qin = mCv (T3 − T2 ) Apply First Law Closed System to Process 4-1,V = Constant Qnet , 41 − Wnet , 41 = ∆U 41 1 Wnet , 41 = Wother , 41 +W b , 41= 0 + ∫ PdV = 0 4 Qnet , 41 = ∆U 41 Qnet , 41 = − Qout = mCv (T1 − T4 ) Qout = − mCv (T1 − T4 ) = mCv (T4 − T1 ) η th , Otto Qout = 1− Qin η th , Otto mCv ( T4 − T1 ) = 1− mCv ( T3 − T2 ) Æ (T4 − T1 ) = 1− (T3 − T2 ) T1 ( T4 / T1 − 1) = 1− T2 ( T3 / T2 − 1) Recall processes 1-2 and 3-4 are isentropic, so T2 ⎛ v1 ⎞ = ⎜⎜ ⎟⎟ T1 ⎝ v2 ⎠ k −1 and T3 ⎛ v4 ⎞ = ⎜⎜ ⎟⎟ T4 ⎝ v3 ⎠ k −1 T2 T3 = T1 T4 Æ v3 = v2 and v4 = v1 or T4 T3 = T1 T2 η th , Otto (T4 − T1 ) = 1− (T3 − T2 ) T1 (T4 / T1 − 1) = 1− T2 (T3 / T2 − 1) 1 η th , Otto T1 = 1− T2 Is this the same as the Carnot efficiency? Efficiency of the Otto Cycle vs. Carnot Cycle • There are only two temperatures in the Carnot cycle • Heat is added at TH • Heat is rejected at TL • There are four temperatures in the Otto cycle!! • Heat is added over a range of temperatures • Heat is rejected over a range of temperatures Since process 1-2 is isentropic, T1 ⎛ V2 ⎞ = ⎜⎜ ⎟⎟ T2 ⎝ V1 ⎠ η th , Otto k −1 = 1 r T1 = 1− T2 Increasing Compression Ratio Increases the Efficiency k −1 Æ η th , Otto = 1 − 1 r k −1 Typical Compression Ratios for Gasoline Engines Why not use higher compression Ratios? • • • • Premature Ignition Causes “Knock” Reduces the Efficiency Mechanically need a better design Diesel Engines • No spark plug • Fuel is sprayed into hot compressed air State Diagrams for the Diesel Cycle Diesel Cycle Otto Cycle The only difference is in process 2-3 Consider Process 2-3 • This is the step where heat is transferred into the system • We model it as constant pressure instead of constant volume qin , 23 − wb ,out = ∆u = u3 − u2 qin , 23 = ∆u + P∆v = ∆h = C p (T3 − T2 ) Consider Process 4-1 • This is where heat is rejected • We model this as a constant v process • That means there is no boundary work q41 − w41 = ∆u q41 = −qout = ∆u = Cv (T1 − T4 ) qout = Cv (T4 − T1 ) As for any heat engine… wnet qout ηth = = 1− qin qin qout = Cv (T4 − T1 ) and qin = C p (T3 − T2 ) nth ,diesel Cv (T4 − T1 ) ( T4 − T1 ) = 1− = 1− k (T3 − T2 ) C p (T3 − T2 ) Rearrange nth ,diesel T1 (T4 T1 − 1) = 1− kT2 (T3 T2 − 1) PV PV 3 3 = 2 2 where P3 = P2 T3 T2 T3 V3 = = rc T2 V2 rc is called the cutoff ratio – it’s the ratio of the cylinder volume before and after the combustion process nth ,diesel T1 (T4 T1 − 1) = 1− kT2 (rc − 1) PV PV 4 4 = 1 1 where V4 = V1 T4 T1 T4 P4 = T1 P1 nth ,diesel T1 (TP44 TP11 − 1) = 1− kT2 (rc − 1) Since Process 1-2 and Process 3-4 are both isentropic PV = P V and k 1 1 k 2 2 P V = PV k 4 4 k 3 3 k ⎛ V3 ⎞ P3 V P4 V k = ⎜⎜ ⎟⎟ = rc = P1 V P2 V ⎝ V2 ⎠ k 3 k 2 k 4 k 1 1 1 nth ,diesel ( ) T1 r − 1 = 1− kT2 (rc − 1) k c Finally, Since process 1-2 is isentropic T1 ⎛ v2 ⎞ = ⎜⎜ ⎟⎟ T2 ⎝ v1 ⎠ T1 ⎛ 1 ⎞ =⎜ ⎟ T2 ⎝ r ⎠ k −1 The volume ratio k −1 from 1 to 2 is the compression ratio, r ηth ,diesel k ⎡ rc − 1 ⎤ 1 = 1 − k −1 ⎢ ⎥ r ⎣ k (rc − 1) ⎦ k=1.4 The efficiency of the Otto cycle is always higher than the Diesel cycle Why use the Diesel cycle? Because you can use higher compression ratios