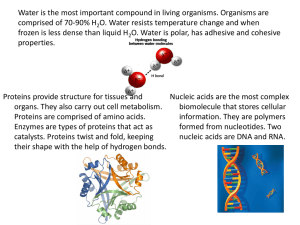
BIOMOLECULES RASHMI KADU ASSISTANT PROFESSOR DEPT. O FORENSIC SCIENCE JAIN (DEEMED-TO-BE UNIVERSITY) • Biochemistry is the study of the chemical processes occurring in living matter. • Biochemists study such things as the structures and physical properties of biological molecules, including proteins, carbohydrates, lipids, and nucleic acids; the mechanisms of enzyme action; the chemical regulation of metabolism; the chemistry of nutrition the molecular basis of genetics (inheritance); the chemistry of vitamins; energy utilization in the cell; and the chemistry of the immune response. • The names of these six elements can be remembered using the acronym chnops. They are not distributed uniformly throughout the body, but some of them concentrate preferentially in some tissues. • Carbon Carbon's ubiquitous nature on earth and beyond lies in its ability to form different types of chemical bonds: single, double and triple. With this property, carbon can join with a wide range of other elements. Carbon is a major component of amino acids, the building blocks of proteins. Proteins, in turn, make up the structural components of most organs and tissues, including muscle, enzymes and neurons. • Hydrogen Hydrogen is the lightest and simplest chemical element, can form only one type of bond – a single bond. Nevertheless, hydrogen can form a greater variety of compounds than any other element, even carbon. It is, as the name implies, found in carbohydrates but also in proteins in fats, which are structural in animals. In addition, the starchy components of plants that give them their shape are made up of carbohydrates. water, which makes up more than two-thirds of the human body, contains hydrogen. • Nitrogen Although nitrogen may get comparatively little attention, it is abundant in nature. More than three-fourths of the earth's atmosphere consists of nitrogen gas. Nitrogen is found in all amino acids and thus in all proteins. In chemical terms, an amino group consists of one nitrogen atom and two hydrogen atoms. While protein is often thought of mainly as a dietary component, proteins are the drivers of everyday life, catalyzing essential biochemical reactions that build the organs and tissues that keep living things growing, adapting and reproducing. • Oxygen Oxygen is vital for respiration on a moment-to-moment basis. At the same time, it is found in water, all proteins and all foods. Fats, which even the leanest animals possess in significant quantities, include oxygen, which – like carbon – is a wondrously versatile molecule from a chemical standpoint. As the earth has aged over the course of its four-billion-plus-year lifetime, the concentration of oxygen in the atmosphere has steadily climbed from trace amounts to about 20 percent, underscoring its crucial nature in the scheme of life. • Phosphorus Phosphorus is something of a background player in the life-maintenance drama. it is a critical part of every plant and animal cell, as it forms the bulk of the phospholipid bilayer that gives cell membranes their integrity while allowing them to be selectively permeable to other substances. phosphorus is also found in bone, and chemical energy derived from metabolic processes is stored for immediate use in phosphorus-based compounds such as ADP (adenosine diphosphate) and ATP (adenosine triphosphate). • Sulfur Sulfur is found in all proteins, most notably in cysteine and methionine. While its role in humans is perhaps not frequently celebrated, it is especially critical in cyclic processes in bacteria, which have been around for billions of years longer than people and will almost certainly be around after human beings are long gone. sulfur is also essential for many bacteria to properly carry out their version of photosynthesis, a set of reactions most commonly associated with plants. Biomolecules Inorganic • Mineral • Gases • Water Organic • Carbohydrates • Lipids • Amino acids • Proteins • Enzymes • Nucleotides • Nucleic acids • Vitamins • MICRO MOLECULES: Small sized, low mol wt between 18 and 800 daltons found in the acid soluble pool. Minerals, gases, water, sugars, amino acids and nucleotides. • MACROMOLECULES: Large sized, high mol wt above 10000 daltons found in the acid insoluble pool. Carbohydrates, lipids, proteins and nucleic acids PROTEINS (C, N, P, O, H, S) • Proteins are class of biologically important compounds. • These are the polymers of amino acid. Amino acids are usually colourless, crystalline solids. These are water-soluble, high melting solids and behave like salts rather than simple amines or carboxylic acids. E.g. Glycine, valine. • Some are hormones (chemical messengers that coordinate biochemical activities in body) eg. insulin • Some act as protective proteins enzymes that fight against foreign substances. eg: gamma globulin • Some transport substances through the organism. eg. hemoglobin. • They might even be fibrous like keratin, or collagen in tendons. • May be found even in toxins/ poisons . • May be essential or non-essential proteins. TYPES OF PROTEINS... • Primary It refers to the number and sequence of amino acid in polypeptide chain. Has amine group on one free end and carboxyl group on the other end of the terminal chain. Eg: lysine and aspartic acid have such chains. • Secondary It helps us to determine the arrangement or the configuration of the structure of the protein. It is of two types: # α-helix: these are right handed coiled structures of proteins. Such kind of proteins can be stretched like elastic. Eg: wool and hair. # Β-helix: these are the kind of proteins in which polypeptide chains are arranged side by side to each other such that they form sheet like structures that can slide over each other. Eg: silk • Tertiary Further folding and bending of the secondary structures at certain places forms tertiary proteins. Eg: insulin • Quaternary It is protein macromolecule formed by interactions between multiple polypeptide chains. Each polypeptide chain is referred to as a subunit. Proteins with quaternary structure may consist of more than one of the same type of protein subunit. Eg: hemoglobin. CARBOHYDRATES (C, H, O) • Carbohydrates are classified on the basis of their behavior on hydrolysis. • They are polyhydroxy compounds having aldehydic or ketonic group. • Generally they are white in colour and are soluble in water. • They are classified into different types depending on whether they undergo hydrolysis or not a) monosaccharaides b) oligosaccharides c) polysaccharides MONOSACCHARAIDES • These are simplest one unit non-hydrolysable sugars. I.E it cannot be broken down to lower sugars. It has only 3 to 9 carbon units. • It has general formula 𝐶𝑛𝐻2𝑛𝑂𝑛 or cx(h2o)y with few exceptions. • Crystalline in nature with sweet taste • About 20 monosaccharide's are known to occur in nature. Some common examples are glucose, fructose, ribose, etc. • The simplest monosaccharide is trioses. • Monosaccharaides are further classified on the basis of number of carbon atoms and the functional group present in them. If a monosaccharide contains an aldehyde group, it is known as an aldose and if it contains a keto group, it is known as a ketose OLIGOSACCHARIDES • These include carbohydrates that form a definite number of monosaccharaide unit on hydrolysis. They may contain 2 to 9 carbon units. • The monosaccharaides units in oligosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage • Disaccharides on hydrolysis with dilute acids or enzymes yield two molecules of either the same or different monosaccharaides.. E.G: sucrose • Trisaccharaides on hydrolysis give three molecules of same or different monosaccharaides. E.G.: Raffinose • Tetrasaccharaides on hydrolysis give four molecules of same or different monosaccharaides. E.G stachyrose POLYSACCHARIDE (NON-SUGARS) • Polysaccharides contain a large number of monosaccharide units joined together by glycosidic linkages. These are the most commonly encountered carbohydrates in nature. They mainly act as the food storage or structural materials. E.G.: Starch, cellulose, insulin etc. • They are colorless, amorphous solids having no taste and insoluble in cold water. OLIGOSACCHARIDE POLYSACCHARIDE MONOSACCHARAIDE LIPIDS (C, H, O) Lipids are molecules that contain hydrocarbons and make up the building blocks of the structure and function of living cells. Examples of lipids include fats, oils, waxes, certain vitamins (such as A, D, E and K), hormones and most of the cell membrane that is not made up of protein. • Lipids types: • Simple: fats & oils waxes • Complex: phospholipids, glycolipids, lipoproteins • Derived: steroids, terpenes Lipids are not soluble in water as they are non-polar, but are thus soluble in non-polar solvents such as chloroform. Lipids that contain an ester functional group are hydrolysable in water. These include neutral fats, waxes, phospholipids, and glycolipids. NUCLEIC ACID • The particles in nucleus of the cell, responsible for heredity, are called chromosomes which are made up of proteins and another type of biomolecules called nucleic acid.. • These are mainly of two types, the deoxyribonucleic acid (dna) and ribonucleic acid (rna). Since nucleic acids are long chain polymers of nucleotides, so they are also called polynucleotides. • Dna (or rna) yields a pentose sugar, phosphoric acid and nitrogen containing heterocyclic compounds (called bases). • Dna contains four bases adenine (a), guanine (g), cytosine (c) and thymine (t). • Rna also contains four bases, the first three bases are same as in dna but the fourth one is uracil (u). • There are three types of rna — mrna, rrna and trna which actually carry out the protein synthesis in the cell. WATER • Medium of biochemical reactions- hydrogen bonding enables water to dissolve many organic biomolecules that contain functional groups which can participate in hydrogen bonding. • Universal solvent - water is a dipole, a molecule with electrical charge distributed asymmetrically about its structure and has a high dielectric constant which enable water to dissolve large quantities of charged compounds such as salts. • Acts as transport medium: many nutrients dissolve in water so that they can be absorbed more easily in your digestive tract. Many metabolic processes take place in water. Water carries waste materials from your cells to your kidneys so that they can be filtered out and eliminated. Many metabolic processes take place in water. • Water is a component of the blood and thus is important for transporting chemicals and nutrients to cells and tissues. Each of your cells is constantly bathed in a watery fluid. Water carries waste materials from your cells to your kidneys so that they can be filtered out and eliminated. • Maintains body temperature: sweat is the main means by which water prevents the human body overheating when the temperature outside it is very high. The evaporation of sweat brings a loss of calories, in the form of heat. This release of energy enables our internal temperature to remain constant. Without this mechanism it would rise in conditions of hot weather or fever. To maintain stable body temperature, we have to both sweat and allow the sweat to evaporate!