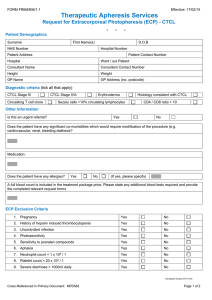
ype of procedure : Therapeutic Photopheresis Number of procedures : 1 Frequency : Other: 2 consecutive procedures every two weeks Duration / over a period off : 3-5 hrs Goals of therapy : Improvement of clinical symptoms by immunomodulation Reason for Therapeutic Photopheresis/Diagnosis : GVHD as complication of bone marrow transplant Whole Blood processed : 1,500 mL Anticoagulant used : ACDA Is patient currently taking anticoagulant medications? : No Change to defined anticoagulation ratio required ? : Yes Is patient taking ACE inhibitors : No Does patient have a contraindication to heparin : No Does patient have an invasive procedure scheduled within 48 : No Type of vascular Access Device : Central Venous Catheter (CVC) Is there a valid consent (Therapeutic Apheresis) : Yes Is there a valid consent (Blood Transfusion) : Yes Patient is 41 yr old Male known case of pemphigus vulgaris since 2016 attended the pre-procedure apheresis clinic for assessment and initiation of apheresis plan and laboratory work for plasma exchange procedure Summary Patient is 41 yr old Male known case of pemphigus vulgaris since 2016 received Immunosuppressive treatment which includes steroids, IVIG, Rituximab, MMF, cyclosporine. Also underwent ECP for 5 months No response/ poor responding Current picture Patient is off steroids except topical on MMF Started Humira (Adalimumab) in May 2022 Patient referred to apheresis for Therapeutic plasma exchange The diagnosis of pemphigus vulgaris is considered a category III Grade 2B by the American society of Apheresis (ASFA) and hence the procedure is advised on case to case basis considering the risks and benefits; as optimum role of apheresis therapy is not established. Case discussed with Dr Abba and Dr Abu Haleeqa. Procedure risks and benefits were explained to the patient. Procedure consents were obtained, signed by the patient and valid. Laboratory parameters including type and screen, CBC, HbS %, Electrolytes, Calcium level, Coagulation profile was ordered /reviewed. Patient has PremCath placed which was checked and procedure will be performed through Prem cath. Calcium gluconate will be administered during the procedure to prevent citrate-induced hypocalcemia. Procedure is planned for 03.06.2022. Plan is to do 5 procedures every other day on OUT patient basis and then reassess and monitor anti desmogelin 1 and 3 antibodies (sent out - to monitor response to treatment) As the patient is on humira (Adalimumab) which is therapeutic mAb. Plasmapheresis and plasma exchange remove circulating proteins from the circulation, including mAbs. Advised to give the humira on the next day of planned TPE procedure. 41 yr Male pemphigus vulgaris since 2016 - Sent out >> anti desmogelin 1 and 3 antibodies titre to monitor response to treatment. received Immunosuppressive treatment as follow managed by dermatology mainly mouth ulcers with decrease oral intake topical treatment ( steroids ) 1) steroids on prednisolone 60mg PO pulses since 2017 2) IVIG since 2018 3) Rituximab 2018 ( 1gm X 2 doses ) 4) MMF 2018 5) cyclosporin ( intolerance ) 6) patient had premCATH in DEC 2021 and then underwnet ECP in ADCC for 5 month ( no improvment ) his Abd levels still high > 160 as per notes Methotrexate , Imuran ( not sure when ) currently off steroids except topical on MMF Started Humira in May 2022 lesions in mouth stable but causing him a lot of discomfort and unable to eat properly with Wt loss followed up by GI JUNE 2 /2022 he was referred by his dermatologist Dr. Zaiedoon for Plasma exchange as his ECP did not work for him ( no clinical improvement ) I explained to him that PLEX procedure is category 2B ( 1 RCT only 40 patient ) and some had benefit but not all and Grade 3 evidence I explained to him the procedure A) he Has PremCath now ( last cleaned 3 weeks ) B) he has to come every other day for 1 month ideally but due to the difficultly will try 2 X a week for at least 1 month reassess in 3month C) we use albumin as replacement fluid usually but sometimes we may need plasma if his coags are affected which can happen with PLEX as we remove his plasma with its antibodies D) I explained the procedure SE ( hypotension , hypocalcemia , allergic reaction bleeding coagulopathy ) I explained to him the benefit targets 1) stable or reduction in pred but will likely stay on it as per most evidence 2) reduction (Antiepdermic Abd) in anti desmogelin 1 and 3 antibodies titre to monitor response to treatment. Which is a sent out test we will do it before and after 1 month of the procedure this is a misclenous test 3) clinically improvement to continue PLAN: 1) Refer to Apheresis Team for assessment for either Plasmapheresis trial 1 month with repeat Abd level Vs re-try ECP in our machine 2) he needs line care 3) FU in 1month to asses response 4) Continue Dermatology FU as per him started humeria still on MMF to continue Patient is a known case of Polycythemia vera referred to apheresis for venesection. Patient has complains of burning sensation feet and hands. o/e ; looks plethoric. 4/7/2022 14:16 UAE 28/6/2022 17:44 UAE Hgb Hct Hgb Hct 162 g/L HI 0.495 L/L HI 152 g/L 0.475 L/L HI Plan; To keep the Hct at 45 percent and the Venesection for 350 ml today. The case was discussed with Dr Husni and the patient attendants in order to consider the possibility of red cell depletion by apheresis, but the attendees refused and opted for simple therapeutic phlebotomy. Procedure risks and benefits were explained to the patient. Procedure consents were valid. Laboratory parameters including CBC was reviewed. Procedure was performed via peripheral line. Good access and flow was obtained . Patient tolerated the procedure well without any adverse events. Patient was under observation for 30 mins after the procedure.