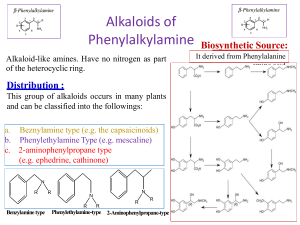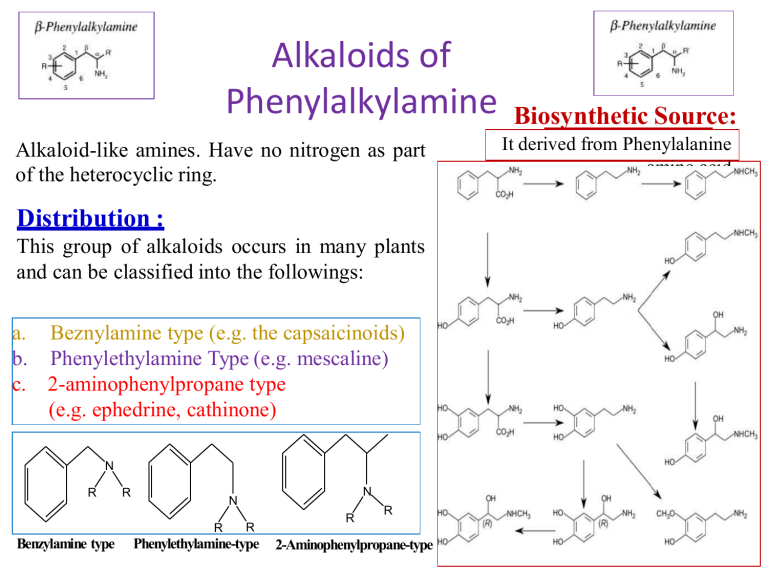
Alkaloids of Phenylalkylamine Alkaloid-like amines. Have no nitrogen as part of the heterocyclic ring. Distribution : This group of alkaloids occurs in many plants and can be classified into the followings: a. Beznylamine type (e.g. the capsaicinoids) b. Phenylethylamine Type (e.g. mescaline) c. 2-aminophenylpropane type (e.g. ephedrine, cathinone) N R R R Benzylamine type N N R Phenylethylamine-type R R 2-Aminophenylpropane-type Biosynthetic Source: It derived from Phenylalanine amino acid Ephedra alkaloids They are present in the arial parts of several Ephedra species, especially Ephedra sinica (Family Ephedraceae). • The plant contains alkaloids, which are phenylethylamine derivatives (derived from l-phenyl alanine), among these (-) ephedrine (l- ephedrine) (1-3%) its stereoisomer (+) pseudoephedrine (d-ephedrine). Psudoephedrine Ephedra sinica d,l-Ephedrine Epinephrine (Adrenaline) Ephedra alkaloids 1- Ephedrine: A-Properties: Ephedrine occurs as solid, steam volatile (i.e. sublimable) alkaloid. It is a strong base (why??), soluble in water. It does not give a precipitate with Mayer's reagent in diluted solution (Gives only with conc solutions). B- Isolation from the plant material: Steam distillation (free base volatile with steam), or by applying any of the usual procedures for extraction of alkaloids. Shaking with dilute HCl, which extracts both ephedrine and pseudo-ephedrine as their hydrochloride salts followed by separation of the mixture. Separation of ephedrine from pseudo-ephedrine: -Ephedrine is more soluble in water than psudo-ephedrine. -Ephedrine oxalate is less soluble in water than psudo-ephedrine oxalate. Ephedra alkaloids C-Tests for identification: Color test: Chen's test (Copper sulphate test): To a solution of ephedrine in water add two drops of 5% CuSO4, and 0.5 ml 20% NaOH, a violet color is produced, on shaking with 1 ml ether or benzene, the organic layer acquires a purple color and the aqueous layer a blue color. Ephedra alkaloids D-Pharmacological action and uses: Ephedrine is an indirectly acting sympathomimetic amine similar to noradrenaline. It is effective orally and has a longer duration of action than noradrenaline (biological catechol amines are rapidly deactivated my COMT enzyme, while ephedra alkaloids are not). COMT Adrenaline In active metabolite Ephedra alkaloids Oral administration of ephedrine causes: -Peripheral vasoconstriction, (α-agonist effect) -Bronchodilator effect (β-agonist effect) -CNS effects (antidepressant and anorexic effect). Psudo-ephedrine can act only as an α-agonist, so it can be use as a peripheral vasoconstrictor (e.g. nasal decongestant), and has very minor CNS effect, so psudo- ephedrine is more safer than ephedrine. Both ephedrine and psudo-ephedrine should be used with caution in Hypertensive patients due to their vasoconstrictive effect. Β-agonist effect -Bronchodilator -CNS stimulant effects. Ephedrine Psudoephedrine α-agonist effect Prepheral vasoconstrictor (Nasal decongestant) Ephedra alkaloids Due to its CNS stimulant effects, and being used as a starting material for the simple synthesis of the more potent CNS stimulant drug (Methamphetamine), ephedrine is regulated as a narcotic drug in many countries worldwide. Ephedrine has been inspired the development of several synthetic drugs For example: -The anorexic drug sibutramine (Meredia®) that has been used for weight control. -Xylometazoline (Otrivin®), that has been used as a topical nasal decongestant. Sibutramine Xylometazoline Alkaloids of the pyridine group Alkaloids belonging to the pyridine group, are subclassified according to the building nuclei into compounds containing: 1.Pyridine nucleus only e.g. Trigonelline from fenugreek. 2.Pyridine nucleus with another nitrogenous ring e.g. tobacco alkaloids. 3.Piperidine nucleus e.g. Pepper, Conium, Lobelia and Pomegranate alkaloids. 4.Pyridone nucleus e.g. Ricinine from castor seeds. Trigonilline Nicotine Coniine Ricinine 1-Fenugreek alkaloids (Pyridine alkaloids) Trigonelline (aka Caffearine) occurs in many seeds as Foenugreek (Trigonella foenum-graecum) and coffee (Coffea arabica). A-Properties: It is , very soluble in water, derived from nicotinic acid (can be semisynthesised from nicotinic acid by methylation), which is biosynthesized from L-tryptophan. It can be easily hydrolysed by HCl, to give methyl chloride and nicotinic acid. B-Uses: Trigonelline is used as a hypoglycemic agent in diabetic patients, as it slows down the metabolism of nicotinic acid, which increase the glucose uptake form blood and its subsequent oxidation. CH3I, (Methylation) HCl (250°C, Hydrolysis) Trigonelline Trigonelline Nicotinic acid 2-Tobacco alkaloids (Pyridine). Tobacco is the cured and dried leaves of Nicotiana tabacum Fam. Solanacea A-Properties: Nicotine possesses two basic nitrogen atoms. It can unite with two molecules of monobasic acids. The weaker basic nitrogen is that in the pyridine ring (Why?). Nicotine decomposes upon exposure to UV light and air to give nicotine oxide ;nicotinic acid and methylamine. Anabasine Nicotelline Nicotine Nor-nicotine 2-Tobacco alkaloids (Pyridine). D-Pharmacological action: Nicotine has a little use in medicine, due to its high toxicity (LD50 = 3 mg/kg, oral), and being highly addictive compound. In small doses, it can act as a respiratory stimulant, and can help in treating nicotine dependence (e.g. nicotine patches, lozenges and sprays). Nicotine can also be used as insecticide in the form of tobacco extract. Nicotine can be used commercially to produce one of a vitamin B- complex named nicotinic acid or Niacin (Vitamin B3) used to prevent Pellagra. 3-Pepper alkaloids (Piperidine alkaloids) In the fruits of pepper species e.g .Piper nigrum Fam. Piperaceae. A-Properties: Piperine is a solid, weak basic alkaloid. It does not form stable salts . It is decomposed easily with alcoholic potash to yield piperidine and piperic acid. B- Uses: Piperine used as condiment . It also used in certain tonic and rubifacient preparations. NaOH, hydrolysis 4-Conium alkaloids (Piperidine alkaloids) In hemlock fruits , Conium maculatum ,Fam. Umbelliferae. The juice from hemlock plant was a primary constituent used by ancient Greeks in executing criminals. An extract of hemlock was used to cause the death of Socrates around 400 BC The major alkaloid of hemlock is coniine. It contains a group of toxic alkaloids of which, coniine and ɣ-coniceine are the main examples. A-Properties: Both coniine and ɣ-coniceine are liquid, volatile with characteristic mice-like odor. D-Pharmacological action and uses: Coniine (LD50 =7 mg/kg, by oral) has a neuromuscular blocking effect. On autonomic ganglia (nicotinic receptor blocker), it produces initial stimulation and subsequent blockage. Coniine has been used as a local analgesic , mostly in external preparations, due to its high toxicity. It is used as an ointment for treatment of hemorrhoids and anal fissures. ɣ-coniceine Coniine 5-Lobelia alkaloids (Piperidine alkaloids) Lobelia herb or Indian tobacco is the dried leaves and flowering tops of Lobelia inflata , Fam. Lobeliaceae (Campanulaceae). B-Pharmacological action and uses: Lobeline produces similar, pharmacological effects as nicotine, however it is not addictive so, lobeline sulfate incorporated in tablets or lozenges, to aid in breaking the tobacco habit (smoke cessation). It is used as antiasthma, respiratory stimulant (bronchodilator) in low dose and as expectorant due to its irritant effect on the stomach. Lobelia inflata Lobelanine l-Lobeline Lobelanidine 6-Pomegranate alkaloids (Piperidine alkaloids) The fruit rind, as well as, the root and stem barks of pomegranate, Punica granatum, Fam. Punicaceae, contain several alkaloids. A-Properties and uses: Usually isolated as a pelletierine tannate. The tannate form of pelletierine is used because it is less soluble in the stomach, so it is less able to be absorbed (local action). Pelletierine Isopelletierine