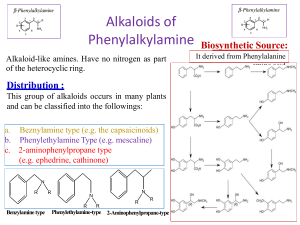
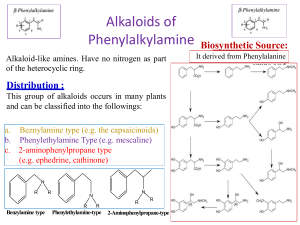
Alkaloids Introduction General Characteristics General Methods of determination of structure Introduction: definition of alkaloid have with as study in alkaloids have progressed Alkaloids means alkali like, basic in nature Originally the name alkaloid was given to all organic bases isolated from plants Konig (1880) suggested alkaloids should be defined as naturally occurring organic bases which contain pyridine ring As per Ladenburg, Alkaloids are natural plant product having basic character and containing at least one nitrogen in heterocyclic ring Considering all the previous definitions Alkaloids may be defined as Organic bases of plant origin, containing a nitrogen heterocycle with strong physiological activity. Most of them are chiral (laevorotatory) Alkaloids are generally solids -Colourless (few are coloured e.g. berberine is yellow) -Crystalline (Some alkaloids e.g. nicotine, coniine liquids soluble in water) -Non Volatile -insoluble in water -Soluble in organic solvents e.g. ethanol, chloroform, ether -Bitter in taste General Methods for determining structure Ziesel Method: It is used to determine the presence and number of methoxyl groups. The alkaloid is heated with conc. Hydriodic acid at its boiling point (126o C). The methoxyl groups are therby converted into methyl iodide, which is then absorbed by ethanolic silver nitrate and silver iodide is weighed Herzig-Meyer Method: It is used to determine the presence and number of N-Methyl group. The alkaloid is heated with conc. Hydriodic acid under pressure ( at 150 to 300o C). The N-Me are therby converted into methyl iodide, which is then absorbed by ethanolic silver nitrate and silver iodide is weighed. Thank You