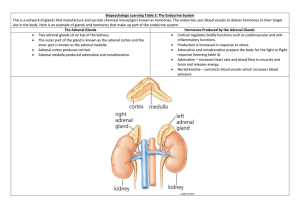
ADRENOCORTICAL HORMONES ANATOMY • The adrenal glands are small, yellowish organs that rest on the upper poles of the kidneys in the Gerota fascia. • The right adrenal gland is pyramidal, whereas the left one is more crescentic, extending toward the hilum of the kidney. • At age 1 year, each adrenal gland weighs approximately 1 g, and this increases with age to a final weight of 4-5 g. • The arterial blood supply comes from 3 sources, with branches arising from the inferior phrenic artery, the renal artery, and the aorta. • Venous drainage flows directly into the inferior vena cava on the right side and into the left renal vein on the left side. • Lymphatics drain medially to the aortic nodes. • Each adrenal gland is composed of two distinct parts: • The adrenal cortex and the adrenal medulla. • The cortex is divided into three zones. • From exterior to interior, these are • The zona glomerulosa, • The zona fasciculata, and • The zona reticularis. Adrenal Cortex • The adult cortex composed of 3 zones – Glomerulosa – Fasciculata – Reticularis 17-Nov-21 Adrenal Cortex Hormones 4 PRODUCTION.. EMBRYOLOGY • First detected at 6 weeks' gestation, the adrenal cortex is derived from the mesoderm of the posterior abdominal wall. • Steroid secretion from the fetal cortex begins shortly thereafter. • Adult-type zona glomerulosa and fasciculata are detected in fetal life but make up only a small proportion of the gland, and the zona reticularis is not present at all. • The fetal cortex predominates throughout fetal life. • The adrenal medulla is of ectodermal origin, arising from neural crest cells that migrate to the medial aspect of the developing cortex. • The fetal adrenal gland is relatively large. • At 4 months' gestation, it is four times the size of the kidney; however, at birth, it is a third of the size of the kidney. • This occurs because of the rapid regression of the fetal cortex at birth. • It disappears almost completely by age 1 year; by age 4-5 years, the permanent adult-type adrenal cortex has fully developed. • Anatomic anomalies of the adrenal gland may occur. • Because the development of the adrenals is closely associated with that of the kidneys, agenesis of an adrenal gland is usually associated with ipsilateral agenesis of the kidney, and fused adrenal glands (whereby the two glands join across the midline posterior to the aorta) are also associated with a fused kidney. • Adrenal hypoplasia occurs in the following two forms: • Hypoplasia or absence of the fetal cortex with a poorly formed medulla • Disorganized fetal cortex and medulla with no permanent cortex present PHYSIOLOGY • Adrenal cortex • The adrenal cortex secretes the following three types of hormones: • Mineralocorticoids (the most important of which is aldosterone), which are secreted by the zona glomerulosa • Glucocorticoids (predominantly cortisol), which are secreted by the zona fasciculata and, to a lesser extent, the zona reticularis • Adrenal androgen (mainly dehydroepiandrosterone [DHEA]), which is predominantly secreted by the zona reticularis, with small quantities released from the zona fasciculata • All adrenocortical hormones are steroid compounds derived from cholesterol Structure Derivative of cholesterol Have 3 cyclohexyl rings( A, B, C) One cyclopentyl ring (D) 18 19 2 10 A 4 12 5 17 16 14 9 B 13 D C 1 3 11 8 15 7 6 Cyclopentanoperhydrophenanthrene 17-Nov-21 Adrenal Cortex Hormones 12 Synthesis Cholesterol ACTH Ang II pregnenolone Cortisol & sex steroids progesterone Deoxycortico sterone 11 hydroxylase Cortisone 18hydroxycortico sterone Aldosterone synthatase 17-Nov-21 Aldosterone synthatase, Ang II Aldosterone Adrenal Cortex Hormones 13 Synthesis Acetate cholesterol pregnenolone 17-OHpregnenolone progesterone 17-OHprogesterone Aldosterone 17-Nov-21 Cortisol Adrenal Cortex Hormones Androgen 14 17-Nov-21 Adrenal Cortex Hormones 15 Cortisol • Cortisol binds to proteins in the blood, mainly cortisolbinding globulin or transcortin. • More than 90% of cortisol is transported in the blood in this bound form. • In contrast, only 50% of aldosterone is bound to protein in the blood. • All adrenocortical steroids are degraded in the liver and predominantly conjugated to glucuronides, with lesser amounts of sulfates formed. • About 75% of these degradation products are excreted in the urine, and the rest is excreted in the stool by means of the bile. Glucocorticoids 17-Nov-21 Adrenal Cortex Hormones 19 Functions of Glucocorticoids • Affect metabolic systems for utilizing – Proteins – Carbohydrates – Fats • 95% of glucocorticoid activity of adrenal cortex result from – Cortisol (hydrocortisone) 17-Nov-21 Adrenal Cortex Hormones 20 Mechanism of Action • Multiple effects of glucocorticoids triggered by – Binding to intracellular receptors which – Interact with specific regulatory DNA sequences • Glucocorticoids Response Element Textbook of Medical Physiology: By Guyton 17-Nov-21 Adrenal Cortex Hormones 21 Mechanism of Action • Induce or repress gene transcription • Leads to Increased or decreased – Formation of mRNA • Alters synthesis of enzymes • Alter cell functions Textbook of Medical Physiology: By Guyton 17-Nov-21 Adrenal Cortex Hormones 22 • Glucocorticoids • Approximately 95% of glucocorticoid activity comes from cortisol, with corticosterone, a glucocorticoid less potent than cortisol, making up the rest. • Normal cortisol concentration in the blood averages 12 μg/dL, with a secretory rate averaging 15-20 mg/day. • Cortisol release is almost entirely controlled by the secretion of ACTH by the anterior pituitary gland, which is controlled by corticotropin-releasing hormone (CRH) secreted by the hypothalamus • In normal situations, CRH, ACTH, and cortisol secretory rates demonstrate a circadian rhythm, with a spike in the early morning. • Various stresses stimulate increased ACTH and, thus, cortisol secretion. • A –ve feedback effect of cortisol on the anterior pituitary and the hypothalamus help control these increases and regulate plasma cortisol concentrations. Glucocorticoids • Produced from zona fasciculata • Cortisol (very potent, accounts for about 95 per cent of all glucocorticoid activity) • Corticosterone (provides about 4 per cent of total glucocorticoid activity, but much less potent than cortisol) • Hydrocortisone (synthetic, almost as potent as cortisol) • Prednisone (synthetic, 4 times as potent as cortisol) • Methylprednisone (synthetic, 5 times as potent as cortisol) • Dexamethasone (synthetic, 30 times as potent as cortisol) • Adrenal hormones are bound to plasma proteins: 90-95% of cortisol in plasma binds to a globulin called cortisol-binding globulin or transcortin & to a lesser extent albumin • Slows elimination giving it a half of 60-90 minutes. While only 60% of aldosterone binds to plasma proteins (40% free form) having a half life of 20 minutes. • Binding serves as a reservoir to lessen rapid fluctuations in free hormone concentration & to ensure uniform distribution to the tissues. Functions of glucocorticoids • 95% of glucocorticoid activity results from cortisol, also known as hydrocortisone. • In addition, there is a small but significant amount of glucocorticoid activity is provided by corticosterone. Carbohydrate metabolism • 1.Stimulation of Gluconeogenesis (formation of carbohydrates from proteins and some other substances) by the liver which is increased as much as 6- to 10- fold. This results mainly from two effects: • Increases the enzymes required to convert amino acids into glucose in the liver cells. • Cause mobilization of amino acids from the extrahepatic tissues mainly from muscle. Results in more amino acids becoming available to enter into the gluconeogenesis process in liver thereby promoting increased formation of glucose. • With increased gluconeogenesis, there is increased glycogen storage in the liver cells allowing other glycolytic hormones, such as epinephrine and glucagon, to mobilize glucose in times of need, such as between meals. • 2.Decreased Glucose Utilization by Cells. • Cause of this is unknown, but believed that somewhere between the point of entry of glucose into the cells and its final degradation, cortisol directly delays the rate of glucose utilization. • 3. Elevated Blood Glucose Concentration and “Adrenal Diabetes.” • Increased rate of gluconeogenesis and reduction in glucose utilization by the cells causes the blood glucose concentration to rise which in turn stimulates insulin secretion. • However, the levels are not as effective in maintaining plasma glucose as they are under normal conditions • High levels of glucocorticoid somehow reduce the sensitivity of many tissues, especially skeletal muscle and adipose tissue, to the stimulatory effects of insulin on glucose uptake and utilization. Protein metabolism • 1.Reduction in Cellular Protein – • Reduces the protein stores in all cells except liver cells. • Caused by both decreased protein synthesis and increased catabolism of protein already in the cells. • Effect results from depressed formation of RNA and subsequent protein synthesis in many extrahepatic tissues, especially in muscle and lymphoid tissue. • In excessive levels cortisol, the muscles can become so weak that the person cannot rise from the squatting position. • 2. Cortisol Increases Liver and Plasma Proteins. • With the reduced proteins elsewhere in the body, liver proteins become enhanced and plasma proteins increased. • This results from the effect of cortisol to enhance amino acid transport into liver cells (but not into most other cells) and to enhance the liver enzymes required for protein synthesis. Fat metabolism • 1.Mobilization of Fatty Acids from adipose tissue. • This increases the concentration of free fatty acids in the plasma, which also increases their utilization for energy. • 2. Obesity Caused by Excess Cortisol – • Moderate degree of fatty acid mobilization from adipose tissue may cause people with excess cortisol secretion to develop a peculiar type of obesity (excess deposition of fat in the chest and head regions of the body, giving a buffalolike torso and a rounded “moon face”). • Although the cause is unknown, it has been suggested that this obesity results from excess stimulation of food intake, with fat being generated in some tissues of the body more rapidly than it is mobilized and oxidized. Cortisol is Important in Resisting Stress and Inflammation • In almost any type of stress, whether physical or neurogenic there is an immediate and marked increase in ACTH secretion, followed within minutes by greatly increased secretion of cortisol. • Some of the different types of stress that increase cortisol release are the following: • Trauma of almost any type • Infection • Intense heat or cold • Injection of norepinephrine and other sympathomimetic drugs • Surgery • Injection of necrotizing substances beneath the skin • Restraining an animal so that it cannot move • Almost any debilitating disease • Significance of this increase is unknown. • However, a possibility is that the glucocorticoids cause rapid mobilization of amino acids and fats from their cellular stores, making them immediately available both for energy and for synthesis of other compounds, including glucose, needed by the different tissues of the body. Anti-inflammatory Effects of High Levels of Cortisol • When large amounts of cortisol are secreted or injected into a person, the cortisol has two basic antiinflammatory effects: • (1) it can block the early stages of the inflammation process before inflammation even begins, or • (2) if inflammation has already begun, it causes rapid resolution of the inflammation and increased rapidity of healing. • These is because cortisol Prevents the Development of Inflammation by Stabilizing Lysosomes and by Other Effects. Cortisol has the following effects in preventing inflammation: • 1. Cortisol stabilizes the lysosomal membranes. • Important because it is makes it more difficult for the membranes of the intracellular lysosomes to rupture. • As such, there is decreased release of proteolytic enzymes that are released by damaged cells to cause inflammation (which are mainly stored in the lysosomes). • 2. Cortisol decreases the permeability of the capillaries, probably as a secondary effect of the reduced release of proteolytic enzymes. This prevents loss of plasma into the tissues. • 3. Cortisol decreases both migration of white blood cells into the inflamed area and phagocytosis of the damaged cells. • These effects are due to diminished formation of prostaglandins and leukotrienes (which normally cause vasodilation, increased capillary permeability, and increased mobility of white blood cells). • 4. Cortisol suppresses the immune system, causing lymphocyte reproduction to decrease markedly. • The T lymphocytes are especially suppressed. In turn, reduced amounts of T cells and antibodies in the inflamed area lessen the tissue reactions that would otherwise promote the inflammation process. • 5. Cortisol attenuates fever mainly because it reduces the release of interleukin-1 from the white blood cells, which is one of the principal excitants to the hypothalamic temperature control system. • The decreased temperature in turn reduces the degree of vasodilation. Cortisol causes resolution of inflammation • Even after inflammation has become well established, the administration of cortisol can often reduce inflammation within hours to a few days. • The immediate effect is to block most of the factors that are promoting the inflammation. • In addition, the rate of healing is enhanced. • This effect of cortisol plays a major role in combating certain types of diseases, such as rheumatoid arthritis, rheumatic fever, and acute glomerulonephritis. • All these diseases are characterized by severe local inflammation, and the harmful effects on the body are caused mainly by the inflammation itself and not by other aspects of the disease. • On administration of cortisol or other glucocorticoids the the inflammation begins to subside within 24 hours. • Glucocorticoids may not correct the basic disease condition but by merely preventing the damaging effects of the inflammatory response, this alone can often be a lifesaving measure. Other effects • Cortisol Blocks the Inflammatory Response to Allergic Reactions • Effect on Blood Cells and on Immunity in Infectious Diseases - Cortisol decreases the number of eosinophils and lymphocytes in the blood; this effect begins within a few minutes after the injection of cortisol and becomes marked within a few hours. • Administration of large doses of cortisol causes significant atrophy of all the lymphoid tissue throughout the body, which in turn decreases the output of both T cells and antibodies from the lymphoid tissue. • This results in decreased immunity to all for almost all foreign invaders • This ability to suppress immunity makes glucocorticoids useful drugs in preventing immunological rejection of transplanted hearts, kidneys, and other tissues. • Cortisol also increases the production of red blood cells (via unclear mechanisms). • Excess cortisol will result in polycythemia and conversely decreased cortisol results in anemia Cellular mechanism of cortisol • Is lipid soluble and will diffuse through the cell membrane. • In cytoplasm, binds with its protein receptor in the cytoplasm • Hormone-receptor complex then interacts with specific regulatory DNA sequences, called glucocorticoid response elements, to induce or repress gene transcription. • Will then increase or decrease transcription of many genes to alter synthesis of mRNA for the proteins that mediate their multiple physiologic effects. • Thus, most of the metabolic effects will not be immediate but require 45 to 60 minutes for proteins to be synthesized, and up to several hours or days to fully develop. • However, especially at high concentrations, they may also have some rapid nongenomic effects on cell membrane ion transport that may contribute to their therapeutic benefits. CORTISOL ON PROTEINS • PRINCIPAL ACTION REDUCTION OF PROTEIN STORES IN ALL TISSUES EXCEPT THE LIVER BY Depressing formation of RNA in most tissues and stimulating protein catabolism CORTISOL ON PROTEINS.. • IN EXCESS • MUSCLE WEAKNESS WHERE ONE CANNOT RISE FROM A SQUATTING POSITION • THIS IS DUE TO INCREASED MOBILISATION OF AMINO ACIDS FROM EXTRAHEPATIC TISSUES DECREASING PROTEIN STORES CORTISOL ON PROTEINS.. • IN EXCESS.. INCREASED PLASMA CONCENTRATION OF AMINO ACIDS LEADS TO 1. INCREASED AMINO ACID DEAMINATION BY LIVER 2. INCREASED LIVER PROTEIN SYNTHESIS 3. INCREASED PLASMA PROTEINS FORMATION BY THE LIVER 4. INCREASED GLUCONEOGENESIS CORTISOL ON FATS • INCREASES Mobilisation of fats from adipose tissue Free fatty acids in the plasma Increasing utilisation FATTY ACID OXIDATION IN CELLS THIS ENHANCES UTILISATION OF FATS INSTEAD OF GLUCOSE IN TIMES OF STARVATION AND OTHER STRESSES RELEASE REGULATION OF CORTISOL SECRETION LEVELS Mineralocorticoids • Aldosterone accounts for 90% of mineralocorticoid activity, with some activity contributed by deoxycorticosterone, corticosterone, and cortisol. • The normal conc’n of aldosterone in the blood ranges from 2-16 ng/dL supine and 5-41 ng/dL upright, although the concentration exhibits diurnal variation, and the secretory rate is generally 150-250 mcg/d. Effects • Promotes Na reabsorption and K+ excretion by the renal tubular epithelial cells of the collecting and distal tubules. • As Na+ is reabsorbed, water follows passively, leading to an increase in the extracellular fluid volume with little change in the plasma Na conc’n. • Persistently elevated extracellular fluid volumes cause hypertension. • This helps minimize further increases in extracellular fluid volume by causing a pressure diuresis in the kidney, a phenomenon known as aldosterone escape. • Without aldosterone, the kidney loses excessive amounts of sodium and, consequently, water, leading to severe dehydration • As Na+ is actively reabsorbed, K+ is excreted. • Imbalances in aldosterone thus lead to hypokalemia and muscle weakness if levels are increased and to hyperkalemia with cardiac toxicity if levels are decreased. • In addition to Na being exchanged for K at the renal tubules, H+ is also exchanged, although to a much lesser extent. • As such, with aldosterone excess, mild metabolic alkalosis may develop. • Also has smaller but similar effect on the sweat glands and salivary glands. • Stimulates NaCl reabsorption and K secretion in the excretory ducts, which helps prevent excessive salivation and conserve body salt in hot climates. • Also affects Na absorption in the intestine, especially the colon. • Deficiency may cause a watery diarrhea from the unabsorbed sodium and water. • Most important factor affecting secretion is the the renin-angiotensin system and changes in the plasma potassium concentration, as follows 1. Activation of the renin-angiotensin system – • The JGA senses decreased blood flow to the kidney secondary to hypovolemia, hypotension, or renal artery stenosis and releases renin • Renin is an enzyme that activates angiotensinogen to release angiotensin I; • In the lungs, ACE converts angiotensin I to angiotensin II, a potent vasoconstrictor and stimulator of aldosterone release by the adrenal gland 2. ECF potassium conc’n – • Increases in the plasma K+ conc’n stimulate the release of aldosterone to encourage potassium excretion by the kidney 3. ECF Na+ Conc’n • Decreases in plasma Na+ conc’n also stimulate aldosterone release 4. Adrenocorticotropic hormone (ACTH) secretion – • ACTH secreted by the anterior pituitary primarily affects release of glucocorticoids by the adrenal but, to a lesser extent, also stimulates aldosterone release Adrenal Androgens • The adrenal cortex continually secretes several male sex hormones which include • Dihdroepiandrosterone (DHEA) • DHEA sulfate (DHEAS), • Androstenedione, and • 11-hydroxyandrostenedione, • Also small quantities of the female sex hormones progesterone and estrogen. • Most of the effects result from extra-adrenal conversion of the androgens to testosterone. • All have weak effects, but they likely play a role in early development of the male sex organs in childhood, and they have an important role in women during pubarche. • ACTH has a definite stimulatory effect on androgen release by the adrenal • Therefore, secretion of these hormones parallels that of cortisol. Adrenal Medulla • The adrenal medulla is a completely different entity. • Epinephrine (80%) • Norepinephrine (20%), and • Minimal amounts of dopamine, • are secreted into the bloodstream due to direct stimulation by acetylcholine release from sympathetic nerves. • Preganglionic sympathetic nerve fibers pass from the intermediolateral horn cells of the spinal cord through the sympathetic chains and splanchnic nerves, without synapsing, into the adrenal medulla. • These hormones are responsible for an increase in cardiac output and vascular resistance and for all the physiologic characteristics of the stress response. Adrenal Pathology • • • • • Glucocorticoid excess Mineralocorticoid excess Androgen excess Catecholamine excess Adrenal insufficiency EFFECTS OF EXCESS PRODUCTION • Glucocorticoid excess or Cushing syndrome • The clinical findings associated with excess cortisol secretion most commonly include • Obesity with moonlike face (the obesity is due to excess deposition of fats in chest and head regions ) • Growth failure, • Hirsutism, and • Acne. Cushing’s syndrome • Corticosteroid dependent cushings syndrome due to excess stimulation by ACTH • Could be due to CRH excess from an extrapituitary tissue eg small cell lung calcinoma. • Could be due to excess secretion of CRH by pituitary corticotroph tumors and is the most common form of the syndrome CUSHING’S SYNDROME... CUSHING’S SYNDROME CUSHING’S SYNDROME CUSHING’S SYNDROME CUSHING’S SYNDROME CUSHING’S SYNDROME • Other findings include • hypertension, muscle weakness, osteoporosis, glucose intolerance, easy bruising, yperpigmentation and thin skin, menstrual irregularities, and psychiatric disturbances. • Patients with cortisol excess also have impaired wound healing and an increased susceptibility to infection. • The differential diagnosis of Cushing syndrome is as follows: • Use of exogenous steroids • ACTH-independent causes - Adrenal nodular hyperplasia, adrenocortical adenoma, adrenocortical carcinoma • ACTH-dependent causes - Pituitary adenoma (Cushing disease), ectopic ACTH (non pituitary tumours producing ACTH) or CRH production from tumors (eg, pancreatic tumor) Mineralocorticoid excess • Presenting features of mineralocorticoid excess include • Hypertension, • Headache, • Tachycardia, • Others are fatigue, proximal muscle weakness, polyuria, and polydipsia. • The differential diagnosis of hyperaldosteronism is as follows: • Primary – • Idiopathic adrenal nodular hyperplasia (idiopathic hyperaldosteronism), glucocorticoid-suppressible hyperaldosteronism, adrenocortical adenoma, adrenocortical carcinoma • Secondary – • Elevated renin secretion secondary to renal artery stenosis, a renin-producing tumor, congestive heart failure, and Bartter syndrome (ie, juxtaglomerular hyperplasia) Primary hyperaldosteronism • • • • • • Characterized by Elevated plasma aldosterone, low plasma renin levels, hypokalemia, and hypertension, Rare in children where, unlike in adults, most common cause is bilateral adrenal hyperplasia • Bilateral adrenal hyperplasia as a cause of hyperaldosteronism occurs in nodular adrenal hyperplasia and in a unique autosomal dominant condition called glucocorticoid-suppressible hyper-aldosteronism. • This has all of the clinical and biochemical features noted in other causes of primary hyperaldosteronism but demonstrates complete and rapid suppression of aldosterone secretion by administration of dexamethasone. Androgen excess • The predominant clinical feature of hyperandrogenism in the newborn girl is ambiguous genitalia. • In the older child or adolescent, signs and symptoms include • pseudoprecocious puberty in boys and • hirsutism, acne, clitoromegaly, deepening of voice, and oligomenorrhea in girls. • In both sexes, linear growth and skeletal maturation (ie, bone age) are accelerated. Causes 1. Use of exogenous anabolic steroids 2. Adrenal causes – • Congenital adrenal hyperplasia , Adrenocortical adenoma, adrenocortical carcinoma, 3. Extra-adrenal causes – • Polycystic ovary, ovarian tumors, testicular tumors (most commonly Leydig cell tumors), adrenal hyperplasia secondary to a pituitary adenoma or ectopic secretion of ACTH or CRH, hyperprolactinemia, acromegaly Catecholamine excess • The clinical manifestations include • hypertension, tachycardia, arrhythmias, headache, fatigue, visual blurring, sweating and heat intolerance, weight loss, abdominal pain, and polyuria and polydipsia. • These symptoms should prompt biochemical testing to confirm excess catecholamine secretion characteristic of pheochromocytoma. • Pheochromocytomas are rare tumors that arise from the neural crest–derived chromaffin cells found in the adrenal medulla and sympathetic ganglia. Adrenal Insufficiency • May be acute or chronic. • Chronic adrenal insufficiency may be primary, secondary, or tertiary. • Acute adrenal insufficiency results when an acute stress is superimposed on chronic adrenal insufficiency of any type. • Symptoms of chronic adrenal insufficiency may be explained by the lack of adrenal hormones and by the unopposed secretion of ACTH. • Hypotension, fatigue, weight loss, anorexia, nausea, vomiting, abdominal pain, salt craving, hypoglycemia, and syncope can occur. • Skin and mucous membrane hyperpigmentation resulting from unopposed secretion of ACTH and melanocyte-stimulating hormone. • Hyponatremia, along with hyperkalemia, is sometimes observed due to chronic insufficiency of aldosterone. • Diagnosis should not be based on the presence or absence of these abnormalities. • The loss of secondary sex characteristics is seen only in women with the disease. Acute Adrenal Insufficiency • Is a medical emergency and must be identified and promptly treated. • Hallmarks of acute adrenal insufficiency are circulatory collapse with abdominal pain that can simulate an acute abdomen. • Profound hypoglycemia, elevated core temperature, and potentially cardiac dysrhythmias are also observed. Chronic adrenal insufficiency • Primary insufficiency • results when the adrenal glands themselves are destroyed or infiltrated • Causes • Congenital adrenal hyperplasia, • bilateral hemorrhage • Severe meningococcal infection or other severe, bacterial infection), TB, HIV, histoplasmosis, and infiltrative diseases (eg, sarcoidosis). • Autoimmune destruction of the adrenal glands is referred to as Addison disease. Secondary insufficiency • Results from diminished release of ACTH from the pituitary. Causes • Trauma, • Pituitary tumors, and • Pituitary hemorrhage (Sheehan syndrome- a condition that affecting women who lose a lifethreatening amount of blood in childbirth or who have severe low blood pressure during or after childbirth. The lack of oxygen from above causes damage to the pituitary gland). Tertiary Insufficiency • Results from suppression of the hypothalamicpituitary-adrenal axis. • Observed with the long-term administration of exogenous steroids. • Important distinguishing feature of tertiary adrenal insufficiency is that adrenal medullary and androgen-secreting functions are preserved.