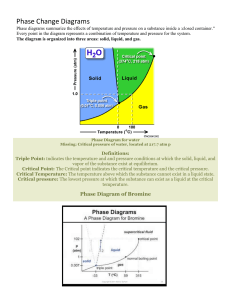
Chapter Outline: Phase Diagrams Microstructure and Phase Transformations in Multicomponent Systems Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited solid solubility) Binary systems with intermediate phases/compounds The iron-carbon system (steel and cast iron) MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 1 Definitions: Components and Phases Component - chemically recognizable species (Fe and C in carbon steel, H2O and Sucrose in sugar solution in water). A binary alloy contains two components, a ternary alloy – three, etc. Phase – a portion of a system that has uniform physical and chemical characteristics. Two distinct phases in a system have distinct physical and/or chemical characteristics (e.g. water and ice, water and oil) and are separated from each other by definite phase boundaries. A phase may contain one or more components. A single-phase system is called homogeneous, systems with two or more phases are mixtures or heterogeneous systems. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 2 Definitions: Solubility Limit Solvent - host or major component in solution, solute minor component. Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e.g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). The same concepts apply to solid phases: Cu and Ni are mutually soluble in any amount (unlimited solid solubility), while C has a limited solubility in Fe. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 3 Microstructure The properties of an alloy depend not only on proportions of the phases but also on how they are arranged structurally at the microscopic level. Thus, the microstructure is specified by the number of phases, their proportions, and their arrangement in space. Microstructure of cast Iron http://www2.umist.ac.uk/material/research/intmic/ (link is dead) The long gray regions are flakes of graphite. The matrix is a fine mixture of BCC Fe and Fe3C compound. Phase diagrams will help us to understand and predict microstructures like the one shown in this page MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 4 Equilibrium and Metastable States A system is at equilibrium if at constant temperature, pressure and composition the system is stable, not changing with time. Equilibrium is the state that is achieved given sufficient time. But the time to achieve equilibrium may be very long (the kinetics can be slow) that a state along the path to the equilibrium may appear to be stable. This is called a metastable state. In thermodynamics the equilibrium is described as a state of a system that corresponds to the minimum of thermodynamic function called the free energy. Thermodynamics tells us that: • The state of stable thermodynamic equilibrium is the one with minimum free energy. • A system at a metastable state is trapped in a local minimum of free energy that is not the global one. Free Energy • Under conditions of a constant temperature and pressure and composition, the direction of any spontaneous change is toward a lower free energy. equilibrium metastable Arrangement of atoms MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 5 Phase diagram Phase diagram is a graphical representation of all the equilibrium phases as a function of temperature, pressure, and composition. For one component systems, the equilibrium state of the system is defined by two independent parameters (P and T), (T and V), or (P and V). Pressure-temperature phase diagram for H2O: MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 6 PVT surface of a pure (1-component) substance http://www.eng.usf.edu/~campbell/ThermoI/ThermoI_mod.html MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 7 A pure substance is heated at constant pressure T Tb V MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 8 Pressure-temperature phase diagram for carbon We can see graphite, diamond, liquid carbon on the phase diagram… but where are fullerenes and nanotubes? MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 9 Phase diagrams for binary systems A phase diagrams show what phases exist at equilibrium and what phase transformations we can expect when we change one of the parameters of the system. Real materials are almost always mixtures of different elements rather than pure substances: in addition to T and P, composition is also a variable. We will limit our discussion of phase diagrams of multicomponent systems to binary alloys and will assume pressure to be constant at one atmosphere. Phase diagrams for materials with more than two components are complex and difficult to represent. An example of a phase diagram for a ternary alloy is shown for a fixed T and P below. ternary phase diagram of Ni-Cr-Fe MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 10 Binary Isomorphous Systems (I) Isomorphous system - complete solid solubility of the two components (both in the liquid and solid phases). L α+L α Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidus line separates solid from liquid + solid MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 11 Binary Isomorphous Systems (II) Example of isomorphous system: Cu-Ni (the complete solubility occurs because both Cu and Ni have the same crystal structure, FCC, similar radii and electronegativity). MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 12 Binary Isomorphous Systems (III) In one-component system melting occurs at a well-defined melting temperature. In multi-component systems melting occurs over the range of temperatures, between the solidus and liquidus lines. Solid and liquid phases are at equilibrium with each other in this temperature range. Liquidus Temperature L liquid solution α+L Solidus liquid solution + crystallites of solid solution α polycrystal solid solution A 20 40 60 80 Composition, wt % MSE 2090: Introduction to Materials Science B Chapter 9, Phase Diagrams 13 Interpretation of a binary phase diagrams For a given temperature and composition we can use phase diagram to determine: 1) The phases that are present 2) Compositions of the phases 3) The relative fractions of the phases Finding the composition in a two phase region: 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Note intersection with phase boundaries. Read compositions at the intersections. The liquid and solid phases have these compositions. XB Xliquid B MSE 2090: Introduction to Materials Science Xsolid B Chapter 9, Phase Diagrams 14 The lever rule Finding the amounts of phases in a two phase region: 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line α+β α β Wα Wβ The lever rule is a mechanical analogy to the mass balance calculation. The tie line in the two-phase region is analogous to a lever balanced on a fulcrum. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 15 Derivation of the lever rule 1) All material must be in one phase or the other: Wα + WL = 1 2) Mass of a component that is present in both phases equal to the mass of the component in one phase + mass of the component in the second phase: WαCα + WLCL = Co 3) Solution of these equations gives us the lever rule. WL = (Cα - Co) / (Cα - CL) Wα = (Co - CL) / (Cα - CL) MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 16 Phase compositions and amounts. An example. Co = 35 wt. %, CL = 31.5 wt. %, Cα = 42.5 wt. % Mass fractions: WL = S / (R+S) = (Cα - Co) / (Cα - CL) = 0.68 Wα = R / (R+S) = (Co - CL) / (Cα - CL) = 0.32 MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 17 Development of microstructure in isomorphous alloys Equilibrium (very slow) cooling MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 18 Development of microstructure in isomorphous alloys Equilibrium (very slow) cooling ¾ Solidification in the solid + liquid phase occurs gradually upon cooling from the liquidus line. ¾ The composition of the solid and the liquid change gradually during cooling (as can be determined by the tie-line method.) ¾ Nuclei of the solid phase form and they grow to consume all the liquid at the solidus line. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 19 Development of microstructure in isomorphous alloys Fast (non-equilibrium) cooling MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 20 Development of microstructure in isomorphous alloys Fast (non-equilibrium) cooling • Compositional changes require diffusion in solid and liquid phases • Diffusion in the solid state is very slow. ⇒ The new layers that solidify on top of the existing grains have the equilibrium composition at that temperature but once they are solid their composition does not change. ⇒ Formation of layered (cored) grains and the invalidity of the tie-line method to determine the composition of the solid phase. • The tie-line method still works for the liquid phase, where diffusion is fast. Average Ni content of solid grains is higher. ⇒ Application of the lever rule gives us a greater proportion of liquid phase as compared to the one for equilibrium cooling at the same T. ⇒ Solidus line is shifted to the right (higher Ni contents), solidification is complete at lower T, the outer part of the grains are richer in the low-melting component (Cu). • Upon heating grain boundaries will melt first. This can lead to premature mechanical failure. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 21 Binary Eutectic Systems (I) systems (alloys) with limited solubility Credit: Kenneth A. Jackson, University of Arizona. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 22 Binary Eutectic Systems (II) Liquidus liquid Temperature, °C Solidus Solvus α+β Copper – Silver phase diagram Composition, wt% Ag Three single phase regions (α - solid solution of Ag in Cu matrix, β = solid solution of Cu in Ag matrix, L - liquid) Three two-phase regions (α + L, β +L, α +β) Solvus line separates one solid solution from a mixture of solid solutions. Solvus line shows limit of solubility MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 23 Binary Eutectic Systems (III) Temperature, °C Lead – Tin phase diagram Invariant or eutectic point Eutectic isotherm Composition, wt% Sn Eutectic or invariant point - Liquid and two solid phases co-exist in equilibrium at the eutectic composition CE and the eutectic temperature TE. Eutectic isotherm - the horizontal solidus line at TE. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 24 Binary Eutectic Systems (IV) Eutectic reaction – transition between liquid and mixture of two solid phases, α + β at eutectic concentration CE. The melting point of the eutectic alloy is lower than that of the components (eutectic = easy to melt in Greek). At most two phases can be in equilibrium within a phase field. Three phases (L, α, β) may be in equilibrium only at a few points along the eutectic isotherm. Single-phase regions are separated by 2-phase regions. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 25 Binary Eutectic Systems (V) Compositions and relative amounts of phases are determined from the same tie lines and lever rule, as for isomorphous alloys •A •B •C For points A, B, and C calculate the compositions (wt. %) and relative amounts (mass fractions) of phases present. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 26 Development of microstructure in eutectic alloys (I) Several different types of microstructure can be formed in slow cooling an different compositions. Let’s consider cooling of liquid lead – tin system at different compositions. In this case of lead-rich alloy (0-2 wt. % of tin) solidification proceeds in the same manner as for isomorphous alloys (e.g. Cu-Ni) that we discussed earlier. L → α +L→ α MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 27 Development of microstructure in eutectic alloys (II) At compositions between the room temperature solubility limit and the maximum solid solubility at the eutectic temperature, β phase nucleates as the α solid solubility is exceeded upon crossing the solvus line. L α +L α α +β MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 28 Development of microstructure in eutectic alloys (III) Solidification at the eutectic composition No changes above the eutectic temperature TE. At TE the liquid transforms to α and β phases (eutectic reaction). L → α +β MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 29 Development of microstructure in eutectic alloys (IV) Solidification at the eutectic composition Compositions of α and β phases are very different → eutectic reaction involves redistribution of Pb and Sn atoms by atomic diffusion. This simultaneous formation of α and β phases result in a layered (lamellar) microstructure that is called eutectic structure. Formation of the eutectic structure in the lead-tin system. In the micrograph, the dark layers are lead-reach α phase, the light layers are the tin-reach β phase. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 30 Development of microstructure in eutectic alloys (V) Compositions other than eutectic but within the range of the eutectic isotherm Primary α phase is formed in the α + L region, and the eutectic structure that includes layers of α and β phases (called eutectic α and eutectic β phases) is formed upon crossing the eutectic isotherm. L → α + L → α +β MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 31 Development of microstructure in eutectic alloys (VI) Microconstituent – element of the microstructure having a distinctive structure. In the case described in the previous page, microstructure consists of two microconstituents, primary α phase and the eutectic structure. Although the eutectic structure consists of two phases, it is a microconstituent with distinct lamellar structure and fixed ratio of the two phases. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 32 How to calculate relative amounts of microconstituents? Eutectic microconstituent forms from liquid having eutectic composition (61.9 wt% Sn) We can treat the eutectic as a separate phase and apply the lever rule to find the relative fractions of primary α phase (18.3 wt% Sn) and the eutectic structure (61.9 wt% Sn): We = P / (P+Q) (eutectic) MSE 2090: Introduction to Materials Science Wα’ = Q / (P+Q) (primary) Chapter 9, Phase Diagrams 33 How to calculate the total amounts of phases? Fraction of α phase determined by application of the lever rule across the entire α + β phase field: Wα = (Q+R) / (P+Q+R) (α phase) Wβ = P / (P+Q+R) (β phase) MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 34 Phase diagrams with intermediate phases Eutectic systems that we have studied so far have only two solid phases (α and β) that exist near the ends of phase diagrams. These phases are called terminal solid solutions. Some binary alloy systems have intermediate solid solution phases. In phase diagrams, these phases are separated from the composition extremes (0% and 100%). Example: in Cu-Zn, α and η are terminal solid solutions, β, β’, γ, δ, ε are intermediate solid solutions. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 35 Phase diagrams with intermetallic compounds Besides solid solutions, intermetallic compounds, that have precise chemical compositions can exist in some systems. When using the lever rules, intermetallic compounds are treated like any other phase, except they appear not as a wide region but as a vertical line. intermetallic compound This diagram can be thought of as two joined eutectic diagrams, for Mg-Mg2Pb and Mg2Pb-Pb. In this case compound Mg2Pb can be considered as a component. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 36 Eutectoid Reactions (I) The eutectoid (eutectic-like in Greek) reaction is similar to the eutectic reaction but occurs from one solid phase to two new solid phases. Invariant point (the eutectoid) – three solid phases are in equilibrium. Upon cooling, a solid phase transforms into two other solid phases (δ ↔ γ + ε in the example below) Looks as V on top of a horizontal tie line (eutectoid isotherm) in the phase diagram. Cu-Zn Eutectoid MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 37 Eutectic and Eutectoid Reactions Temperature l γ Eutectoid temperature α γ +l l +β Eutectic temperature γ +β α+γ β α +β Eutectoid composition Eutectic composition Composition The above phase diagram contains both an eutectic reaction and its solid-state analog, an eutectoid reaction MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 38 Peritectic Reactions A peritectic reaction - solid phase and liquid phase will together form a second solid phase at a particular temperature and composition upon cooling, e.g. L + α ↔ β Temperature These reactions are rather slow as the product phase will form at the boundary between the two reacting phases thus separating them, and slowing down any further reaction. α + liquid α +β β liquid β + liquid Peritectics are not as common as eutectics and eutectiods, but do occur in some alloy systems. There is one in the FeC system that we will consider later. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 39 Congruent phase transformations A congruent transformation involves no change in composition (e.g., allotropic transformation such as α-Fe to γ-Fe or melting transitions in pure solids). For an incongruent transformation, at least one phase changes composition (e.g. eutectic, eutectoid, peritectic reactions). Congruent melting of γ MSE 2090: Introduction to Materials Science Ni-Ti Chapter 9, Phase Diagrams 40 The Iron–Iron Carbide (Fe–Fe3C) Phase Diagram In their simplest form, steels are alloys of Iron (Fe) and Carbon (C). The Fe-C phase diagram is a fairly complex one, but we will only consider the steel part of the diagram, up to around 7% Carbon. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 41 Phases in Fe–Fe3C Phase Diagram ¾ α-ferrite - solid solution of C in BCC Fe • Stable form of iron at room temperature. • The maximum solubility of C is 0.022 wt% • Transforms to FCC γ-austenite at 912 °C ¾ γ-austenite - solid solution of C in FCC Fe • The maximum solubility of C is 2.14 wt %. • Transforms to BCC δ-ferrite at 1395 °C • Is not stable below the eutectoid temperature (727 ° C) unless cooled rapidly (Chapter 10) ¾ δ-ferrite solid solution of C in BCC Fe • The same structure as α-ferrite • Stable only at high T, above 1394 °C • Melts at 1538 °C ¾ Fe3C (iron carbide or cementite) • This intermetallic compound is metastable, it remains as a compound indefinitely at room T, but decomposes (very slowly, within several years) into α-Fe and C (graphite) at 650 - 700 °C ¾ Fe-C liquid solution MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 42 A few comments on Fe–Fe3C system C is an interstitial impurity in Fe. It forms a solid solution with α, γ, δ phases of iron Maximum solubility in BCC α-ferrite is limited (max. 0.022 wt% at 727 °C) - BCC has relatively small interstitial positions Maximum solubility in FCC austenite is 2.14 wt% at 1147 °C - FCC has larger interstitial positions Mechanical properties: Cementite is very hard and brittle can strengthen steels. Mechanical properties also depend on the microstructure, that is, how ferrite and cementite are mixed. Magnetic properties: α -ferrite is magnetic below 768 °C, austenite is non-magnetic MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 43 Classification. Three types of ferrous alloys ¾ Iron: less than 0.008 wt % C in α−ferrite at room T ¾ Steels: 0.008 - 2.14 wt % C (usually < 1 wt % ) α-ferrite + Fe3C at room T Examples of tool steel (tools for cutting other metals): Fe + 1wt % C + 2 wt% Cr Fe + 1 wt% C + 5 wt% W + 6 wt % Mo Stainless steel (food processing equipment, knives, petrochemical equipment, etc.): 12-20 wt% Cr, ~$1500/ton ¾ Cast iron: 2.14 - 6.7 wt % (usually < 4.5 wt %) heavy equipment casing MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 44 Eutectic and eutectoid reactions in Fe–Fe3C Eutectic: 4.30 wt% C, 1147 °C L ↔ γ + Fe3C Eutectoid: 0.76 wt%C, 727 °C γ(0.76 wt% C) ↔ α (0.022 wt% C) + Fe3C Eutectic and eutectoid reactions are very important in heat treatment of steels MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 45 Development of Microstructure in Iron - Carbon alloys Microstructure depends on composition (carbon content) and heat treatment. In the discussion below we consider slow cooling in which equilibrium is maintained. Microstructure of eutectoid steel (I) MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 46 Microstructure of eutectoid steel (II) When alloy of eutectoid composition (0.76 wt % C) is cooled slowly it forms perlite, a lamellar or layered structure of two phases: α-ferrite and cementite (Fe3C) The layers of alternating phases in pearlite are formed for the same reason as layered structure of eutectic structures: redistribution C atoms between ferrite (0.022 wt%) and cementite (6.7 wt%) by atomic diffusion. Mechanically, pearlite has properties intermediate to soft, ductile ferrite and hard, brittle cementite. In the micrograph, dark areas are Fe3C layers, light phase is α-ferrite MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 47 Microstructure of hypoeutectoid steel (I) Compositions to the left of eutectoid (0.022 - 0.76 wt % C) hypoeutectoid (less than eutectoid -Greek) alloys. γ → α + γ → α + Fe3C MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 48 Microstructure of hypoeutectoid steel (II) Hypoeutectoid alloys contain proeutectoid ferrite (formed above the eutectoid temperature) plus the eutectoid perlite that contain eutectoid ferrite and cementite. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 49 Microstructure of hypereutectoid steel (I) Compositions to the right of eutectoid (0.76 - 2.14 wt % C) hypereutectoid (more than eutectoid -Greek) alloys. γ → γ + Fe3C → α + Fe3C MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 50 Microstructure of hypereutectoid steel MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 51 Microstructure of hypereutectoid steel (II) Hypereutectoid alloys contain proeutectoid cementite (formed above the eutectoid temperature) plus perlite that contain eutectoid ferrite and cementite. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 52 How to calculate the relative amounts of proeutectoid phase (α or Fe3C) and pearlite? Application of the lever rule with tie line that extends from the eutectoid composition (0.75 wt% C) to α – (α + Fe3C) boundary (0.022 wt% C) for hypoeutectoid alloys and to (α + Fe3C) – Fe3C boundary (6.7 wt% C) for hipereutectoid alloys. Fraction of α phase is determined by application of the lever rule across the entire (α + Fe3C) phase field. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 53 Example for hypereutectoid alloy with composition C1 Fraction of pearlite: WP = X / (V+X) = (6.7 – C1) / (6.7 – 0.76) Fraction of proeutectoid cementite: WFe3C = V / (V+X) = (C1 – 0.76) / (6.7 – 0.76) MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 54 The Gibbs phase rule Let’s consider a simple one-component system. In the areas where only one phase is stable both pressure and temperature can be independently varied without upsetting the phase equilibrium → there are 2 degrees of freedom. P liquid solid gas T Along the lines where two phases coexist in equilibrium, only one variable can be independently varied without upsetting the two-phase equilibrium (P and T are related by the Clapeyron equation) → there is only one degree of freedom. At the triple point, where solid liquid and vapor coexist any change in P or T would upset the three-phase equilibrium → there are no degrees of freedom. In general, the number of degrees of freedom, F, in a system that contains C components and can have Ph phases is given by the Gibbs phase rule: F = C − Ph + 2 MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 55 The Gibbs phase rule – example of a binary system F = C − Ph + 2 P = const F = C − Ph + 1 C=2 F = 3 − Ph ¾ In one-phase regions of the phase diagram T and XB can be changed independently. ¾ In two-phase regions, F = 1. If the temperature is chosen independently, the compositions of both phases are fixed. ¾ Three phases (L, α, β) are in equilibrium only at the eutectic point in this two component system (F = 0). MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 56 Summary Make sure you understand language and concepts: ¾ Austenite ¾ Cementite ¾ Component ¾ Congruent transformation ¾ Equilibrium ¾ Eutectic phase ¾ Eutectic reaction ¾ Eutectic structure ¾ Eutectoid reaction ¾ Ferrite ¾ Gibbs phase rule ¾ Hypereutectoid alloy ¾ Hypoeutectoid alloy ¾ Intermediate solid solution ¾ Intermetallic compound ¾ Invariant point ¾ Isomorphous ¾ Lever rule ¾ Liquidus line ¾ Metastable ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ ¾ MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams Microconstituent Pearlite Peritectic reaction Phase Phase diagram Phase equilibrium Primary phase Proeutectoid cementite Proeutectoid ferrite Solidus line Solubility limit Solvus line System Terminal solid solution Tie line 57