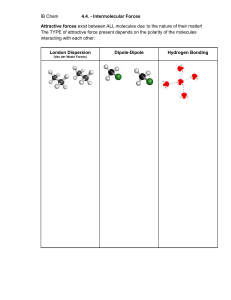
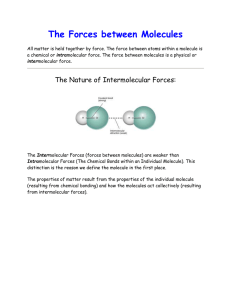
General Chemistry 2 11 General Chemistry 1 – Grade 11 Quarter 3 – Module 1: Types of Intermolecular Forces First Edition, 2020 Republic Act 8293, section 176 states that: No copyright shall subsist in any work of the Government of the Philippines. However, prior approval of the government agency or office wherein the work is created shall be necessary for exploitation of such work for profit. Such agency or office may, among other things, impose as a condition the payment of royalties. Borrowed materials (i.e., songs, stories, poems, pictures, photos, brand names, trademarks, etc.) included in this module are owned by their respective copyright holders. Every effort has been exerted to locate and seek permission to use these materials from their respective copyright owners. The publisher and authors do not represent nor claim ownership over them. Published by the Department of Education Division of Pasig City Development Team of the Module Writer: Roanna A. Cabigting Editor:Ma. Victoria G. Señase Reviewer: Liza A. Alvarez Illustrator: Edison P. Clet Layout Artist: Micaelle Lauren V. Tenorio Management Team: Ma. Evalou Concepcion A. Agustin OIC-Schools Division Superintendent Carolina T. Revera, CESE OIC-Assistant Schools Division Superintendent Manuel A. Laguerta EdD OIC-Chief, Curriculum Implementation Division Education Program Supervisors Librada L. AgonEdD(EPP/TLE/TVL/TVE) Liza A. Alvarez(Science/STEM/SSP) Bernard R. Balitao(AP/HUMSS) Joselito E. Calios (English/SPFL/GAS) Norlyn D. CondeEdD(MAPEH/SPA/SPS/HOPE/A&D/Sports) Wilma Q. Del Rosario (LRMS/ADM) Ma. Teresita E. HerreraEdD(Filipino/GAS/Piling Larang) Perlita M. IgnacioPhD(EsP) Dulce O. SantosPhD(Kindergarten/MTB-MLE) Teresita P. TagulaoEdD(Mathematics/ABM) Printed in the Philippines by Department of Education – Schools Division of Pasig City General Chemistry 2 11 Quarter 3 Module 1 Types of Intermolecular Forces Introductory Message For the facilitator: Welcome to the General Chemistry 1 Quarter 3 Module 1 Intermolecular Forces! on the Types of This module was collaboratively designed, developed, and reviewed by educators from Schools Division Office of Pasig City headed by its Officer-In-Charge Schools Division Superintendent, Ma. Evalou Concepcion A. Agustin in partnership with the Local Government of Pasig through their mayor, Honorable Vico Sotto. The writer utilized the standards set by the K to 12 Curriculum using the Most Essential Learning Competencies (MELC) while overcoming their personal, social, and economic constraints in schooling. This learning material hopes to engage the learners in guided and independent learning activities at their own pace and time. Furthermore, this also aims to help learners acquire the needed 21st-century skills especially the 5 Cs namely: Communication, Collaboration, Creativity, Critical Thinking, and Character while taking into consideration their needs and circumstances. In addition to the material in the main text, you will also see this box in the body of the module: Notes to the Teacher This contains helpful tips or strategies that will help you in guiding the learners. As a facilitator you are expected to orient the learners on how to use this module. You also need to keep track of the learners' progress while allowing them to manage their own learning. Moreover, you are expected to encourage and assist the learners as they do the tasks included in the module. For the learner: Welcome to the General Chemistry 1 Quarter 3 Module 1 on the Types of Intermolecular Forces! The hand is one of the most symbolized parts of the human body. It is often used to depict skill, action, and purpose. Through our hands, we may learn, create, and accomplish. Hence, the hand in this learning resource signifies that you as a learner is capable and empowered to successfully achieve the relevant competencies and skills at your own pace and time. Your academic success lies in your own hands! This module was designed to provide you with fun and meaningful opportunities for guided and independent learning at your own pace and time. You will be able to process the contents of the learning material while being an active learner. This module has the following parts and corresponding icons: Expectations - This points to the set of knowledge and skills that you will learn after completing the module. Pretest - This measures your prior knowledge about the lesson at hand. Recap - This part of the module provides a review of concepts and skills that you already know about a previous lesson. Lesson - This section discusses the topic in the module. Activities - This is a set of activities that you need to perform. Wrap-Up - This section summarizes the concepts and application of the lesson. Valuing - This part integrates a desirable moral value in the lesson. Posttest – This measures how much you have learned from the entire module. EXPECTATIONS This module is developed and designed for Senior High School STEM students. This lesson is about the types of intermolecular forces. Specifically, you are expected to: 1. describe the types of intermolecular forces; 2. differentiate the types of intermolecular forces; and, 3. recognize the importance of types of intermolecular forces in daily life. PRE–TEST Direction: Choose the letter of the best answer. 1. Which of the intermolecular forces exists between molecules of I2? A. Dipole-dipole B. Hydrogen bonding C. Ion-ion interaction D. London dispersion 2. Which A. B. C. D. of the following choices would mean interaction between molecules? Coulomb`s Law Electrostatic force Intermolecular force Intramolecular forces 3. For which of the following molecules apply dipole-dipole interactions? A. CH4 C. CCl4 B. C6H6 D. CH2Cl2 4. Which of these molecules will NOT form hydrogen bonds with water? A. HF C. NH3 B. CH4 D. HCOOH 5. Which of the following types of intermolecular forces is the weakest? A. Dipole-dipole B. Hydrogen bonding C. Ion-ion interaction D. London dispersion RECAP You may find out that some of the questions are not familiar, don’t get disappointed because this module is designed for you to make the topic easier to understand. Let us have a recap first! Direction: Complete the table below. Describe the type of bonding that occurs in the compound. Compound Molecular Formula Bond Type 1. ammonia 2. carbon tetrabromide 3. sodium chloride 4. carbon dioxide 5. water So, how about the forces that keep between molecules? Ok, walkthrough on these forces that keep them together. let us have a L E S S ON “How do coronavirus spread?” There are many factors that lead to the acing spread of coronavirus. It could be transmitted from person to person via droplets, contact, and fomites. When one sneezes or coughs droplets of saliva containing the COVID-19 virus are then inhaled by another person. It is so sad to note that COVID-19 transmission usually occurs among close contacts which affects our family members. Therefore, it is important to maintain a distance of more than 1 meter away from any person who has respiratory symptoms. The closer we are with this kind of virus the greater the chance we get infected leading to a worst effect in our health. Likewise, in chemistry, if a molecule is exposed or placed near to another molecule the tendency is that they get attracted and build up a force that will hold them together. The force between these molecules will greatly affect some of their physical properties such as melting point and boiling points. What do you think are these forces that hold together one molecule to another molecule? We call these forces as intermolecular forces. Ok, let us have a walkthrough on these intermolecular forces. Types of Intermolecular Forces (IMF) The interaction between molecules ( substances that are made of atoms that bond through the sharing of electrons to form covalent bonds) are governed by physical forces called intermolecular forces. These are forces that arise from the way in how electrons are shared within the covalent bonds of different molecules. These types of forces also affect the physical properties of compounds which will be further explained in this module. The following are the different types of intermolecular forces: A. Ion Ion Interaction What are ions? How does it build an attraction with the other ions? Ions are charged particles or have permanent whole number charges. Remember that like charges repel each other and opposite charges attract, as shown in figure 1. The attraction between these ions are pulled together by a force called an electrostatic force. Remember that electrostatic force as stated in Coulomb`s Law is directly proportional to the charge of the ions and inversely related to the distance between them. The equation is also shown in figure 1. Figure 1 How do we determine the strength of the built forces between ions? As shown in the equation, the strength of the electrostatic force that is built depends on the product of the charges (Z1Z2) divided by the square of the distance of separation (d2). Meaning that as the particles (ions) get attracted the force between these ions becomes stronger as they get closer. The attraction between these ions is called ion-ion interaction. Let us have examples by comparing the molecules below. Which from these ions will have a stronger ion-ion interaction? According to the equation given in the figure above, the higher the magnitude of the charges the higher is the electrostatic force, so the interaction between Ca+2 and O-2 ions is stronger than the Na+ and Cl- ions. Why? Because the charges in calcium and oxygen are higher than the sodium and chloride ions. In the case of ions with the same charges, this time you have to take note of the size of the ions because as the size of the ion increases the electrostatic force decreases. The physical property such as the melting point of a compound is greatly affected by the magnitude of the lattice energy or the electrostatic energy built between the ions. Meaning that the higher the electrostatic force between molecules will have a higher melting point. To illustrate this, let us compare Aluminum nitride and Magnesium oxide, wherein aluminum nitride will have a higher magnitude of charge which is +3 for aluminum and -3 for nitrogen, while magnesium oxide is lower having +2 for magnesium and -2 charge for oxygen. Therefore, the molecule that has a higher melting point is aluminum nitride. B. Ion Diplole Interaction Ion dipole interaction is very evident when pouring water molecules around sodium ions which is the case when dissolving sodium chloride in water. Figure 3 Water is a permanent dipole molecule because it has positive and negative poles as a result of the uneven distribution of electrons within it. So when a molecule has two opposite partial charges they are dipole and polar. Once water molecules surround the sodium ions, the oxygen that is partially negative in the water molecule will be attracted to the sodium ion which is positively charged. Eventually, the positively charged hydrogen in the water molecule will be attracted to the chloride ion. C. Dipole-dipole Interaction From the given compounds above, which do you think is a dipole molecule, and why? Yes, all of the given compounds are all dipole molecules. What makes them dipole is because of the partial opposite charges present in the molecule. When we say dipole-dipole interaction, we are referring to the interaction of the two dipole molecules such as between molecules of carbon monoxide, hydrochloric acid, and nitrogen trifluoride. Wherein the two poles of each molecule is either partially positive or partially negative. So let us have an example to illustrate this type of interaction. Figure 4 Figure 4 shows the molecules of nitrogen trifluoride where fluorine is more electronegative than nitrogen. And once a molecule of nitrogen trifluoride reacts with another molecule of nitrogen trifluoride, the partially negative fluoride ions will get attracted to the partially positively charged nitrogen of another nitrogen trifluoride molecule. The attraction between the opposite charges is called dipole-dipole interaction. And if we put in another nitrogen trifluoride, this molecule will rearrange itself in such a way that the partial positive of the nitrogen in this molecule is attracted to the partially positive on the fluorines of the other nitrogen trifluoride molecule. The same thing will happen to the molecules of hydrochloric acid and carbon monoxide which are shown below in figure 5 and figure 6. Figure 5 Figure 6 D. Hydrogen Bond Interaction What do you think is the most obvious similarities among the molecules below? The given above molecules exhibit a special kind of dipole-dipole interaction that occurs specifically between a hydrogen atom bonded to either an oxygen, nitrogen, or fluorine atom. Meaning that the hydrogen is partially positive and is attracted to the nitrogen, oxygen, and fluorine which are partially negative. The strength of hydrogen bonding is relatively strong that requires energy to break it. No wonder why water molecules have high boiling points and melting points. Hydrogen bonding also plays a vital role in holding the nucleotide bases together in our DNA and RNA. Take note that you might be claiming the bond between hydrogen and fluorine atoms is a hydrogen bond. It is not, because if we say the intermolecular type of interaction, we are considering the attraction between molecules not within the molecule. Figure 7 and figure 8 show the hydrogen bonding for the ammonia and hydrofluoric acid. Figure 7 Figure 8 The strength of the hydrogen bonding depends on the extensiveness or number of formed hydrogen bonds and the polarity of the bond. The arrangement of the strength of the hydrogen bond is H-O< H-N <H-F because H-F is a highly polar molecule. E. London dispersion Interaction / Van der Waals Intermolecular Forces This type of interaction happens to be present in all types of molecules whether ionic or covalent-polar or nonpolar. However, this type of intermolecular force is significant in nonpolar molecules and the force is developed due to the uneven distribution of electrons and create a temporary dipole. This type of force is a very weak type of dipole interaction. The force between these molecules increases with the polarizability (squishiness of a molecule), molecular size (more electrons), and pi bonding (overlapping of orbitals). Figure 9 The above figure 9 shows the increasing size of the molecules from the halogen group. So from the molecules above the iodine molecule would have a stronger London dispersion force. London dispersion force (LDF) is responsible for the liquid phase of noble gases. Figure 9 shows how helium gets into the liquid phase. The figure shows that originally we have two atoms of Helium. But once these two atoms get closer, the electrons outside the nucleus of helium will migrate in such a way that the electrons of the other helium atom will get attracted to the proton of the other helium. This attraction between opposite charges is the Coulombs`s Law, thus creating a temporary dipole. The London dispersion force is simply the connection between these two atoms. Figure 10 Arrangement on the strength of force present among the types of intermolecular forces is shown in figure 11. Figure 11 Among these types, the strongest type of intermolecular forces is ion-ion dipole because of the permanent charge while London dispersion forces are the weakest because of the presence of temporary dipole and they are usually in gas forms. Strong intermolecular forces increase the physical behavior of the molecules such as melting points, boiling points, viscosity, and surface area. Use these facts on the types of intermolecular forces in answering the following activities. ACTIVITIES Activity 1 Direction: Analyze each of the following statements whether it describes the types of intermolecular forces. Write TRUE if the statement is correct but if it’s false, change the underlined word or group of words to make the whole statement true. Write your answer on a separate sheet of paper. 1. London dispersion is evident in nonpolar molecules. 2. Hydrogen bonding refers to a hydrogen from one molecule boded to N, F, and Br to the other molecule. 3. Ion-dipole interaction leads to the building of forces between nonpolar molecules. 4. According to ion-ion interaction, the electrostatic force increases as the size of the magnitude of charges decreases. 5. Dipole-dipole interaction is significant between an ion and a dipole molecule. Now, use these facts on types of intermolecular forces in answering Activity 2. Activity 2 Direction: Complete the table by writing all types of intermolecular forces (IMF) that will exhibit between the pairs of molecules. Pairs of molecules 1. O=C=O and O=C=O 2. Type(s) IMF 3. 4. 5. Let us have a try out on how well is your understanding in analyzing the importance of applying these types of intermolecular forces in our daily life. Do activity 3. Activity 3 Direction: Rank the following molecules according to the stated physical property. 1. Rank the following in terms of increasing boiling point: CaBr2 C2H5OH C4H10 _________________________________________________________________________________ 2. Rank these substances from lowest to highest intermolecular forces: HF F2 PCl3 __________________________________________________________________________________ 3. Rank the following in terms of increasing melting point: MgCl2 NaCl AlCl3 __________________________________________________________________________________ WRAP–UP Direction: Complete the table by describing the types of intermolecular forces and arrange these types according to increasing strength by numbering them 1-5. Types of Intermolecular Forces Io-ion interaction Ion-dipole interaction Diopole-dipole interaction Hydrogen bond London dispersion force Description of the types of Intermolecular Forces Strength of Intermolecular Forces VALUING Just like molecules, humans are also attracted to one another. As a teenager, you have experienced being attracted to someone. How did you deal with your attractions to others? Do you listen to your parents for advice on this matter? Finally, you made it! Take your last step and good luck! POST TEST Direction: Choose the letter of the best answer and write your answer on a separate sheet of paper. 1. What type(s) of intermolecular forces can exist between HF and H2S? A. B. C. D. Hydrogen bonding London dispersion Dipole-dipole Ionic bonding 2. What type(s) of intermolecular forces can exist between I2 and CBr4? A. B. C. D. Hydrogen bonding London dispersion Dipole-dipole Ionic bonding 3. What type(s) of intermolecular forces can exist C2H6 and BF3? A. B. C. D. Dipole-induced dipole Hydrogen bonding London dispersion Ionic bonding 4. Which of the following will form a hydrogen bond with NH 3? A. H2O B. CH4 C. Cl2 D. CO2 5. Which of the following shows the increasing boiling points of halogens? A. F2 < I2 < Br2 < Cl2 B. I2 < Cl2 < Br2 < F2 C. Cl2 < Br2 < I2 < F2 D. F2 < Cl2 < Br2 < I2 KEY TO CORRECTION Recap 1. NH3 - Covalent polar 2. CBr4 - Nonpolar covalent 3. NaCl - Ionic Pretest 1. C 2. C 3. D 4. B 5. D 1. LDF only 2. LDF and Ion-dipole 3. LDF and dipole-dipole 4. LDF only (H2 has no H-bond acceptor) 5. LDF and ion-dipole Activity 2 Activities Activity 1 1. TRUE 2. Oxygen 3. Polar 4. Increases 5. Dipole molecules Wrap Up 1. Melting point (high,low) 2. Boiling point (High,low) 3. Surface Area (High,low) 4. Molecular size (High, low) Posttest 1. A 2. B 3. A 4. A 5. D 3. NaCl < MgCl2 < AlCl3 2. F2 < PCl3 < HF Activity 3 1. C4H10 < C2H5OH < CaBr2 REFERENCES Andersen. 2013. London dispersion force. August 13. https://www.youtube.com/watch?v=1iYKajMsYPY. Mindtouch. 2020. Atomic_and_Molecular_Properties/Intermolecular_Forces/Specific_Interactions/Io. May 18. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_M aps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_ Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Specific_Interactions/Io. N, Truong-Son. 2018. why-are-intermolecular-forces-important. July 5. https://socratic.org/questions/why-are-intermolecular-forces-important. specialist, Khanacdemy. 2020. intramolecular-and-intermolecular-forces. https://www.khanacademy.org/science/class-11-chemistry-india/xfbb6cb8fc2bd00c8:in-instates-of-matter/xfbb6cb8fc2bd00c8:in-in-intermolecular-forces/a/intramolecular-andintermolecular-forces. Winegrad, Jake. 2017. Ion-Ion(Intermolecular Forces). March 6. https://www.youtube.com/watch?v=1zhyHv2NJ04.