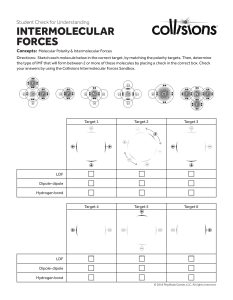
Intermolecular Forces Intra vs Inter The intermolecular forces of attraction between particles determine whether a substance is a solid, liquid or gas at room temperature. Intermolecular attractions are generally weak when compared to intramolecular bonds, but span a wide range of strengths. Types of Intermolecular forces London Dispersion Forces Dipole-Dipole Forces Hydrogen Bonding London Dispersion The London dispersion force is a temporary attractive force that results when the electrons in two adjacent NONPOLAR atoms occupy positions that make the atoms form temporary dipoles. Remember (+) and (-) attract Dipole-Dipole Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. What happens if you still push them together? https://phet.colorado.edu/sims/html/atomic-interactions/latest/atomic-interactions_en.html Hydrogen Bonding A hydrogen bond is a primarily electrostatic force of attraction between a hydrogen atom which is covalently bound to a more electronegative atom or group, and another electronegative atom bearing a lone pair of electrons https://www.youtube.com/watch?v=UukRgqzk-KE https://www.youtube.com/watch?v=HVT3Y3_gHGg Volatility Boiling point relations