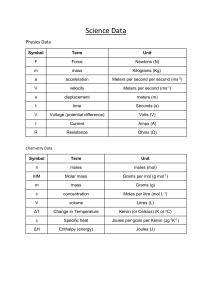
Stoichiometry Diagnostic 2 – Answers Mass to Mass (using a balanced chemical equation and molar masses) 1. Given 35.0 grams of silver (Ag), find the mass of silver nitride (Ag3N) that would be produced. Also find the mass of magnesium nitride (Mg3N2) you would need for this reaction to go to completion. 6 Ag + Mg3N2 -> 2 Ag3N + 3 Mg Molar masses: Ag = 107.87g/mol, Ag3N = 337.62g/mol, Mg3N2 = 100.95g/mol Step 1: Mass Ag into moles Ag = 35.0g x (1 mol/107.87g) = moles Ag Step 2: Moles Ag into moles Ag3N = moles Ag x (2 moles Ag3N/6 moles Ag) = moles Ag3N Step 3: Moles Ag3N into mass Ag3N = moles Ag3N x (337.62g/mol) = g Ag3N Step 4: Moles Ag (Step 1) into moles Mg3N2 = moles Ag x (1 mole Mg3N2/6 moles Ag) = moles Mg3N2 Step 5: Moles Mg3N2 into mass Mg3N2 = moles Mg3N2 x (100.95g/mol) = g Mg3N2 2. Given 15.2 grams of lithium hydroxide (LiOH), find the mass of both magnesium hydroxide (Mg(OH)2) and lithium oxide (Li2O) that would be produced. MgO + 2 LiOH -> Mg(OH)2 + Li2O Molar Masses: LiOH = 23.95g/mol, Mg(OH)2 = 58.33g/mol, Li2O = 29.88g/mol Step 1: Mass LiOH into moles LiOH = = Step 2: Moles LiOH into moles = = Step 3: Moles into mass = = Step 4: Moles LiOH (Step 1) into moles = = Step 5: Moles into mass = = 3. Say you wanted to make 10.0 grams of sodium sulfide from aluminum sulfide and sodium chloride. a) Write the balanced chemical equation modelling this reaction. Al2S3 + 6 NaCl -> 3 Na2S + 2 AlCl3 b) Find the moles of sodium sulfide in 10.0 grams. = 10.0 grams Na2S x (1 mol/78.04g) = 0.128139 moles Na2S c) Find the mass of aluminum sulfide you would need for this reaction. Step 1: moles Na2S to Al2S3 moles = 0.128139 mol Na2S x (1mol Al2S3/3mol Na2S) = 0.042713 moles Al2S3 Step 2: moles Al2S3 to mass Al2S3 = 0.042713 moles Al2S3 x (150.14g/mol) = 6.41 grams of Al2S3 d) Find the mass of sodium chloride you would need for this reaction. Step 1: moles Na2S to moles NaCl = 0.128139 mol Na2S x (6 mol NaCl/3 mol Na2S) = 0.256278 moles NaCl Step 2: moles NaCl to mass NaCl = 0.256278 moles NaCl x (58.44g/mol) = 14.98 grams of NaCl Limiting and Excess Reagent 4. Given ___ grams of ___ and ___ grams of ___, as well as the balanced chemical formula: a) How many grams of ___ can you make? b) Which reactant was limiting? c) How much extra will you have of your excess reagent? 5. Given ___ grams of ___ and ___ grams of ___, as well as the balanced chemical formula: a) How many grams of ___ can you make? b) Which reactant was limiting? c) How much extra will you have of your excess reagent? Percent Composition d) Find the percent composition by mass for each atom in 500 grams of C6H12O6 = e) Find the percent composition by mass for each atom in 155 grams of H2SO4 = Empirical Formula f) Given a percent composition of: Find the empirical formula g) Given a percent composition of: Find the empirical formula Molecular Formula h) Given a percent composition of: a) Find the empirical formula b) If the mass of 1 mole of sample is ___, what is the molecular formula? i) Given a percent composition of: c) Find the empirical formula d) If the mass of 1 mole of sample is ___, what is the molecular formula?