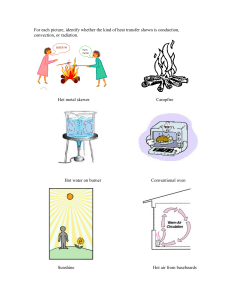
HEAT TRANSFER Success Criteria By the end of this this Chapter, you must be able to: 1. Explain how heat is transferred from one object to another 2. Compare the rate of conduction of heat by different metals 3. Explain application of heat transfer in everyday life Key words: heat, conduction, convection, radiation, thermal flask, insulation HEAT AND TEMPERATURE Heat • Heat is the form of energy which passes from the body of high temperature to the body of low temperature. • The SI unit of heat is joule (J). Temperature • Temperature is the degree of coldness or hotness of a substance. • Kinetic Theory of Matter states that particles of matter are always in motion. • Temperature can be defined as a measure of the average kinetic energy of the molecules of a substance. • The SI unit for temperature is Kelvin (K). • But the common measure of temperature is in degrees Celsius (oC). Differences between heat and temperature i. Heat is a form of energy while temperature is the degree of hotness and coldness. ii. Heat is measured in joules (J) while temperature is measured in degrees Celsius (oC). Experiment on heat and temperature Aim: To investigate the difference between heat and temperature Apparatus/materials: a measuring cylinder, a beaker, cooking oil, two test tubes, a stirrer and water Procedure: 1. Take equivalent masses of water and cooking oil in two identical test tubes fitted with two identical thermometers and place the test tubes in a large beaker containing water. 1 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com 2. Note and record the initial temperature of both water and oil in the tubes. 3. Heat the water in the beaker making sure that there is even distribution of heat by stirring the water. 4. After sometime. Note the temperature of water and oil in the tubes. Observation The temperature of water is lower than that of oil. Discussion • The test tubes received the same heat energy and the same heat energy has been passed from the burner to the test tubes. • Both oil and water have gained the equal amount of heat energy but are at different temperature. • Therefore, two test substances can have equal heat energy supplied but at different temperatures rise to different values. Conclusion • Heat is the form energy while temperature is the degree of hotness and coldness of a substance. HEAT TRANSFER IN SOLIDS, LIQUIDS AND GASES. Activity Materials: a charcoal burner or candle, a wire or a bicycle spoke, a pot or a small tin and some water. Procedure: 1. Light the charcoal burner or candle and leave it for about a minute. 2. Sit close to the charcoal burner or candle. What do you feel? 3. Now place one end of the wire or bicycle spoke above the flame of the charcoal burner or candle. • How do you feel? Leave it for some time. How do you feel? 4. Now pour water into the pot. 5. Place the tin on the charcoal burner. 2 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com 1. At intervals touch the water with your hand, how do you feel after doing this for a number of times? Observation You should be able to feel hot in all the instances. a) When you sat close to the candle or burner you felt warm after some time showing that the heat was transferred through the air (gas) to your body. b) When you were holding the wire above the charcoal burner, your hand eventually felt hot such that you could not keep holding the wire. This shows that heat was being transferred through the wire (solid). c) In the third instance, you felt the water getting warmer and warmer until the water felt so hot that you could not touch it anymore. This means the heat was transferred through the water (liquid). Conclusion These results show that heat can be transferred through solids, liquids as well as gases. MODES OF HEAT TRANSFER There are three methods of heat transfer namely: A. Conduction B. Convection C. Radiation A. CONDUCTION • Conduction is the method of heat transfer in solids whereby heat is transferred from particle to particle. • Conduction occurs from region of high temperature to the region of low temperature. • There is no visible movement of the heated particles. Experiment Aim: to demonstrate conduction of heat in solids Apparatus/materials: Copper rod and Bunsen burner Procedure: 1. Light a bunsen burner and hold the copper with the other end in a bunsen burner flame as shown below. 2. Observe what will happen after sometime Fig: Heat moves from the hot end to the cold end of a metal pole 3 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Results/observation • After sometime you feel heat in your hand until the copper rod becomes too hot to hold. Explanation/discussion • The copper rod gets hot because heat is transferred from the side which is in a bunsen burner flame to where you are holding. Conclusion • Therefore, solids transfer heat from one point to another. Mechanism of conduction of heat in solids • When the molecules at one end of a solid receive heat energy from the heat supply, they begin to vibrate vigorously. • These molecules collide against the neighbouring molecules and agitate them. • The agitated molecules, in turn, agitate the molecules in the next layer and so on till the molecules at the other end of the solid are agitated. • Thus, the heat is passed from one point to another till the other end becomes hot. • Hence, in conduction, energy transfer takes place by vibration of the molecules. • There is no actual movement of the heated particles. Types of conductors a. Good conductors b. Bad conductors c. Non-conductors or insulators A. Good conductors • These are materials that conduct heat well. • They are made up of atoms which hold their electrons loosely sot that some of the electrons are free to move. The electrons carry heat. • A good conductor is a good emitter of heat. Examples are copper, aluminium, and iron. B. Bad conductors • These are materials that conduct heat poorly. Examples silicon, water, air and glass C. Non-conductors or insulators • These are materials that do not conduct heat. • Their atoms do not have free electrons. Examples plastics, rubber, wood, paper and cork 4 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com To demonstrate that heat energy flows due to a temperature difference Materials: • An iron bar about a metre long with holes drilled at equal intervals • Oil • Thermometer • Wooden screen • Water bath • A bunsen burner • Hammer • Metal punch Procedure 1. Fill the holes of the iron bar partially with oil and insert the bulb of the thermometers into them. Note the readings of the thermometers. 2. Heat one end of the iron bar slowly and gradually (Fig. 5.4). Observe the temperature increase in the thermometers and record the readings. Fig. The higher the temperature difference the higher the energy transferred 3. After some time, note the temperature readings of the thermometers. What do you observe? Explain. Observation • The thermometer nearest to the hot bunsen burner registers the highest rise in temperature, and the one farthest away registers the least temperature rise. • Initially the readings of all the thermometers were the same. When one end of the rod was inserted into boiling water, a large temperature difference was set up between the two ends and heat energy flowed from the region of higher temperature to that of lower temperature. • Hence heat energy flows due to temperature difference. • If the activity is repeated by replacing the hot water bath with a bunsen burner flame (temperature of the bluish part of the flame is about 500˚C), the rise in temperature registered by each thermometer is higher. Hence the higher the temperature difference, the higher the energy transfer. 5 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com • Conclusion Heat energy flow in solids is due to temperature difference. The higher the temperature difference, the higher the energy flow. Comparing rate of conduction in metals Aim: to compare conductivity of heat in solids Apparatus/materials: copper rod, aluminium rod, iron rod, wax, 3 match sticks, tripod sand, bunsen burner Procedure: 1. Take three rods, say copper, aluminium and iron of the same size and length and fix a match stick to one end of each rod using melted wax. 2. Place the rods on a tripod stand and heat the free ends with a burner as shown below. 3. Observe what happens. Observation • The match stick falls off from copper rod first then aluminium and finally from the iron rod. Discussion • The match stick do not fall off at the same time because the energy transferred is not equal for all the three rods. • The match stick from the copper rod is the first one to fall off showing that of the three metals, copper is the best conductor of heat followed by aluminium and then iron. Conclusion • Different materials conduct heat at different rates. Experiment Aim: To demonstrate that water is a bad conductor of heat Apparatus/materials: a piece of ice, a boiling tube, bunsen burner, wire gauze, stand Procedure 1. Wrap the piece of ice in a wire gauze and drop it into a boiling tube containing water. 2. Heat the water with a bunsen burner flame as shown below until it boils. 6 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Observation/results • The water at the top of the tube boils. The ice at the bottom does not melt easily even after heating for a long time. Discussion • The ice is failing to melt because heat is not being conducted easily by water from the top to the bottom. Conclusion Water is a poor conductor of heat. Factors affecting heat transfer through conduction Temperature differences. • Heat energy in solids flow due to differences in temperature. The higher the temperature difference, the higher the energy flow or the higher the rate of transfer. ii. Material differences. • Different materials conduct heat at different rates. iii. Thickness/ cross section • Thick materials conduct heat faster than thin materials. (the larger the cross-sectional area the higher the transfer). iv. Length/size differences. • Short materials conduct heat faster than long materials. v. Duration of heating. • Time taken to heat the material will determine how much heat is conducted though the material. i. Relative conductivities • Different substances at room temperature conduct heat differently. • The conductivity of heat in air at room temperature is said to be 1. • The heat conduction of different substances related to heat conduction in air at room temperature is called relative conductivity. • Some of the relative conductivities are as follows; 7 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Substance Air Wood Cardboard Brick Water Glass (window) Conductivity 1 6 8 23 25 35 Substance Mercury Iron Brass Aluminium Copper Silver Conductivity 270 3000 4500 8000 16000 18000 Application of conduction of heat Conduction of heat is used in the following situations a. Used as conductors b. Used as insulators 1. Used in making cooking pots: cooking pots are made of metals to conduct heat transfer. 2. Used in making irons for ironing clothes 3. Used in making cooking pots handles: the handles of cooking pots are made of plastic or wood because they are insulators. 4. Used in making tea cups: most tea cups are made of insulators 5. Used in clothing: air trapped next to the skin reduces heat loss on a very cold day. Likewise, it minimises heat gain on a hot day. Note that air is a bad conductor of heat 6. It is used in wall insulation: in cold weather, countries minimise heat loss or gain by making the wall of the house with two sets of bricks with a gap between them. The gap is filled with an insulating material. 7. Roof insulation: grass thatched houses trap air in between the grass. Since glass is a good insulator, it prevents loss or gain of heat inside the house during adverse weather B. CONVECTION • Convection is a mode of heat transfer through fluids by actual physical movement of molecules of the fluids due to temperature differences within the fluid. • During convection of heat, the warmer and less dense part of the fluid moves upwards and the colder and denser part of the fluid moves falls to the place of the warmer and less dense part • Stream of a liquid or a gas when heated are called convection currents. • In fluids transfer of heat by convection is faster than through conduction. • Liquids and gases are fluids. Investigations on heat transfer through fluids Aim: to observe convection current in water Apparatus/materials: long straw, bunsen burner, a beaker containing water, a crystal of permanganate, flask Procedure 8 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com 1. Drop a piece of a crystal of potassium permanganate to the centre of the bottom of the flask or a beaker containing water using a long straw. 2. Heat the flask gently at the centre and observe what happens. Fig. Convection currents in water Observation Coloured streaks are observed to rise from the bottom to the top. Discussion • The crystal dissolves and the hot water of less density starts rising displacing the cold dense water down. • The streams of physically moving warm liquid are called convection currents Conclusion • Heat energy is transferred by the convection currents in the liquid Investigations on heat transfer through gases Aim: to observe convection current in air Apparatus/materials: a box with glass window and two chimneys, candle, smouldering pieces of wick Procedure 1. Take a box with a glass window and two chimneys fixed at the top. 2. Place a lighted candle under chimney A and hold a smouldering piece of wick above chimney B 3. Observe what happens. 9 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Observation • Smoke from the smouldering wick is seen to move down through chimney B then to the candle flame and finally comes out through chimney A. Discussion • Air above candle flame on becomes warm and its density decreases. Warm air rises up through chimney A and the cold dense air above chimney B is drawn down this chimney and passes through the box and up the chimney A. • The smoke particles from the wick enable us to see path of convection current Conclusion • Heat is transferred in air through convection currents. Convection current possesses energy Experiment Aim: to illustrate that convection current possesses energy Apparatus/materials: thin circular disk, cardboard, candle flame Procedure 1. Take a thin circular disk of tin or cardboard and cut six blades all round. 2. Pivot the disk on a bent needle. 3. Hold the disk above the candle flame for some time. 4. Observe what happens. 10 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Fig. A rotating disk Observation • The disk start to rotate. Discussion • The rotation is due to the convection current set up. • . If a powerful electric bulb is available, you can make a rotating lamp shade. Fig. A rotating lamp shade Conclusion • Convection current possesses energy. Application of convection of heat Convection is used in the following situations; 1. Brings about the land and sea breezes. 2. Used as land and sea breezes 3. Used for ventilation 4. Used in hot water system 5. Used for cooling car engines 6. Used in convector heater 7. Used in immersion water heaters by placing them at the bottom. C. RADIATION • Radiation is the method of heat transfer in a vacuum. • All objects give off heat by radiation 11 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com • • The heat that is being transferred is called radiant. At higher temperature more radiant energy (heat) is emitted and the low temperature less heat is emitted. • In radiation, heat transmission does not require a medium. For example, energy from the sun across the empty spaces beyond the earth’s atmosphere. • The radiation energy consists of invisible electromagnetic waves that are able to pass through a vacuum. How heat is transferred through radiation • When radiation falls on an object, it is partly reflected, partly transmitted and partly absorbed. • When an object on earth absorbs radiation from the sun, the absorbed radiation is transferred to internal energy called heat energy. Factors affecting radiation i. Temperature of the body. • Increase in temperature increase amount of energy radiated. ii. Colour of the body. • Dull colours such as black absorb more heat energy than bright colours such as white. iii. Type of conductor. • Bad conductors are good absorbers and good emitters while good conductors are poor absorbers and poor emitters. Investigations on radiation Aim: investigation on heat transfer through radiation Apparatus/materials: wax, a thumb tack, bunsen burner, a thin tin lid pained black Procedure 1. Take a thin tin lid painted black on one side. Stick a thumb tack with melted wax on the other side. 2. Keep the bunsen burner flame close to the painted side. Fig. Radiation Observation The wax melts and the thumb tack falls off 12 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Discussion • The energy from the flame reaches the tin lid and the wax by a different mode called radiation • The amount of heat energy radiated depends upon the temperature of the body. In the activity above, if the bunsen burner is replaced by a candle flame, it will take a longer time for the wax to melt. • The temperature of the candle flame is lower than that of a bunsen burner. Conclusion Heat transfer can take place without contract or in a vacuum Effect of Wearing Dark Coloured Clothes. Good and bad absorbers of heat energy by radiation Experiment 1 Materials: • Two thin tin lid • A metal thumb tack (match stick) • Molten wax • A bunsen burner Procedure 1. Take two thin tin lids, one with the inner side shiny and the other with the inner side painted dull black. 2. Stick metal thumb tacks (or match sticks) on the outside of each lid using a little molten wax. 3. Keep a bunsen burner flame midway between the lids as shown in figure below 4. Watch closely to and compare what happens to the two thumb tacks. Explain your observation. Fig. Good and bad absorbers Observation • The thumb tack on dull black surface will fall off first than on a shiny 13 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com surface discussion • If a black and shiny surface receive the same amount of heat energy by radiation, the black surface absorbs more heat than the shiny surface. • A dull black surface is a better absorber of heat radiation than a shiny surface Experiment 2 Aim: To investigate good and bad absorbers Materials: a black paint, a white or shiny paint, two metallic tins, two pieces of cardboard and two thermometers. Procedure: 1. Paint one of the tins black and the other one white. 2. Make small hole on each piece of cardboard so that the thermometer can fit. 3. Insert the thermometer into each hole of the cardboard. 4. Place the cardboard with the thermometer on top of each tin such that the bulb of the thermometer is inside the tin as you can see in the image below. 5. Place both tins in the sun for some time. 6. Observe the temperature changes and record them at regular intervals in the table like the one below. 7. You may record the data in a table like the one below. Tin Colour Initial Temperature Final Temperature Black White Observation • You might be able to notice that the temperature rises quickly in the thermometer placed inside the black tin than the one in the white or shiny tin. • The thermometers registered different temperatures after being exposed to the same amount of heat at the same period of time. • Similarly, rough surfaces radiate more heat than smooth or polished surfaces. • This is what happens when you wear a black shirt on a hot day. • The black shirt gets hotter faster than a white or shiny one. • If you let these tins cool, you will see that the black one also cools down faster. • This means dark coloured clothes will also make you feel cold on a cold day. 14 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Conclusion Dark objects are good conductors (absorbers) of heat than white or shiny objects. Solar panel • For the fact that black surfaces absorb more heat, that’s why the solar panel surfaces are painted black. • The black surfaces are the collectors hence they absorb a lot of heat as they are black. Fig. The solar cells. The black surfaces are the collectors hence they absorb a lot of heat as they are black. To illustrate good and bad emitters of heat Aim: to illustrate good and bad emitters Apparatus/materials: three thermometers, three cardboard, three identical empty tins. Procedure 1. Take three identical cans of the same volume without tops 2. Paint one white the other black (both inside and outside) and leave the third can shiny. 3. Prepare the three suitable cardboard covers with holes at the centre. 4. Fill the cans to the brim with hot water at 600C 5. Cover the cans with cardboards and place a thermometer in each can through the hole at the centre. 6. Record the temperature of water in the cans after a certain time interval. 15 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Observation • The water in the can painted black cools the fastest. The water in the shiny can takes the longest time to cool. Conclusion • Dull black surfaces are good emitter of heat while shiny surfaces are bad emitter of heat. Law of radiation • Law of radiation states that a black surface is a good absorber of heat and a good emitter (radiator) of heat while a shiny surface is a poor absorber and a poor emitter (radiator) of heat radiation. Application of radiation 1. Electric kettles have a chrome coat to reduce radiation. 2. Electric iron are silver coated to minimize radiation. 3. Green houses use radiation (heat trap) to grow crops. 4. Clouds reflect radiation back to the earth hence cloudy nights are warmer than clear nights. 5. Construction domestic hot water system. 6. Extraction of solar energy. 7. Construction of solar heater. 8. Construction of solar concentrations. 9. Construction of glass thatched houses Effect of painting of walls white • • • • • In many of the houses especially in the regions that experience varied temperature changes, the walls are painted white. If not white, then other light or bright colours are used. The white colour reflects heat to prevent it entering the house on a hot day. This ensures that the occupants of the house still feel cool on a hot day. On a cool day, the white walls reflect the heat into the house thereby preventing it from going outside the house on a cool day. 16 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com A B In figure A inside of a house that has been painted white to prevent heat from going outside on a cold day. In figure B the house has been painted white outside. This prevents entry of heat into the house on hot days such that people inside the house can still feel cool even when the day is hot. The use of aluminium foil in: keeping food warm; designing simple solar cookers; designing heaters. Food warmer • • Food warmer keeps food warm for a longer time" It is made of a shiny aluminium. This aluminium reflects heat back into the warmer thereby ensuring that the heat stays in the warmer hence keeping the food warm for a long time. Fig. In this image you can see the shiny silver made of aluminium inside a food warmer Solar cookers • • • Aluminium is also used to make solar cookers. The solar cooker is made of aluminium surfaces that help to reflect heat. The reflected heat is collected onto the cooking pot and the heat is used to cook lighter foods. 17 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Fig. This solar cooker is made of aluminium which reflects light onto a pot and it is converted to heat which helps cook the food. The green house • The sun emits radiation inform of light with short wavelength infrared which easily passes through glass without being absorbed. • The rays are absorbed by the earth and objects inside the green house, which in turn, raise the temperature of the air by conduction and convection • Therefore, greenhouse becomes very hot and acts as a radiation trap. The vacuum flask Fig. A thermos flask: This flask has the double silver glass as well a vacuum seal. How a thermos flask works • The thermos flask serves to keep hot liquids hot or cold liquids cold for a long period. • The silver glass is painted shiny silver in order to reflect heat as well as prevent its loss or entry in case of hot or cold liquids respectively. • The silver glass is a double glass with a vacuum inside. • This prevents conduction of heat as well as convection current creation. 18 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com • The vacuum seal prevents entry of air into the space between the double glass thereby maintaining the vacuum. Why a flask do lose heat Due to design problems, the flask will slowly loses heat because 1. Radiation through the vacuum still take place; only that it is minimised. 2. There is radiation and convection at the upper part of the liquid, so heat is transferred from the liquid to the closing cap, which in turn conducts the heat to the outside. Refrigerator • The refrigerator is painted white in order to prevent absorption or emission of heat because it is a poor absorber or a poor emitter of heat radiation • This keeps the refrigerator cool at all times. Thatched houses • • Thatched houses have a very uniform (not changing frequently) temperature since grass is a poor conductor of heat hence preventing heat entry and exit in the house. Summer hats and cottages are thatched using grass since grass has a high specific heat capacity thereby insulating the hats and keeping them cool even in hot weather. Fig: a grass-thatched house. Cooler box • • Inside the cooler box it is white, this ensures that the contents of the cooler box should not gain heat easily. It has also has some insulating material to prevent heat transfer thereby maintaining the temperature of the contents of the cooler box 19 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Fig: Cooler box Land and sea breeze a. Land breeze At night, both the land and the sea lose heat. The land loses heat much faster than the sea. This causes the air above the sea to rise because it is less dense (lighter). Cold air blows from the land to the sea to replace the risen air. Fishermen at night at the lake fell a cool breeze blowing from the land. Fig. Land breeze b. Sea breeze Sea breeze occurs during the day. The land heats up more quickly than the sea. This causes the air above the land to rise because it is less dense (lighter). Cooler denser air from the sea moves to the land to replace the rises hot air. If you stand by the sea side during the day, you will feel a cool breeze from the sea this is sea breeze because hot air blows from the sea to the land. 20 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Fig. Sea breeze Ventilation • Ventilation is the supply of fresh air to a room or building. • Fresh cold air enters a building through the windows placed low down near the floor. • The air becomes warm in the house and the warm air rises and escapes through ventilation holes high up on the walls or in the ceilings as in figure below. Fig. Ventilation in a room Hot water system • The boiler heats the water, the water rises by convection up to the hot water storage tank. • The cooler water in the tank sinks down to the boiler. • In this way a supply of hot water collects in the storage tank. • The pressure needed to force hot water out of the taps is provided by a header tank on the roof. • A valve called a ball cock controls the supply of water into the tank. • If the water level drops, the ball cork opens; as the water level rises, it shuts again. • The expansion pipe is a safety precaution. • If the water boils, the expansion pipe shoots steam into the header tank. 21 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com Cooling car engines • The engine is covered with a metal water jacket. • The metal conducts heat from the engine and heats the water. • The water jacket is connected to a radiator. • The radiator is cooled by outside air flowing past it. • The engine is covered with a metal water jacket. • The metal conducts heat away from the engine and heats the water. • The water jacket is connected to a radiator. • Most cooling systems uses pumps to help convection currents (make use of forced convection). • Cooling of motor car engines is one of such systems. Convector heater • The electric elements heat the air which rises out of the top of a case. • Cold air flows in at the bottom to replace hot air. 22 Compiled by Owen Kaipa Musyani 0999 066 560/0884 209 888 musyanio@gmail.com