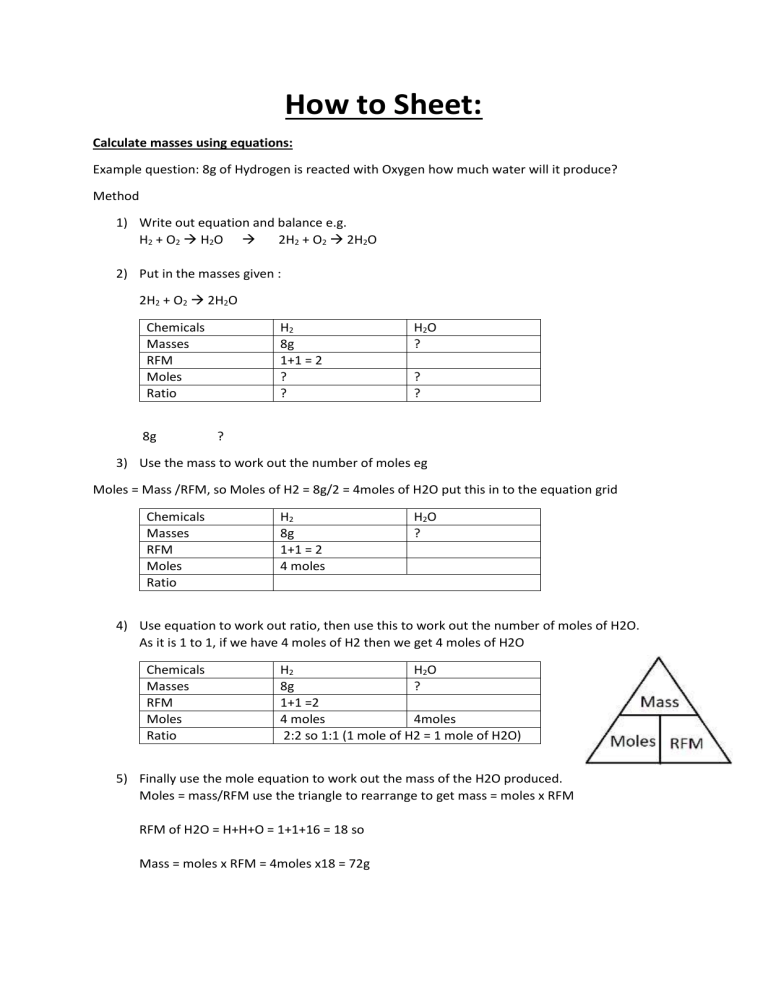
How to Sheet: Calculate masses using equations: Example question: 8g of Hydrogen is reacted with Oxygen how much water will it produce? Method 1) Write out equation and balance e.g. H2 + O2 H2O 2H2 + O2 2H2O 2) Put in the masses given : 2H2 + O2 2H2O Chemicals Masses RFM Moles Ratio 8g H2 8g 1+1 = 2 ? ? H2O ? ? ? ? 3) Use the mass to work out the number of moles eg Moles = Mass /RFM, so Moles of H2 = 8g/2 = 4moles of H2O put this in to the equation grid Chemicals Masses RFM Moles Ratio H2 8g 1+1 = 2 4 moles H2O ? 4) Use equation to work out ratio, then use this to work out the number of moles of H2O. As it is 1 to 1, if we have 4 moles of H2 then we get 4 moles of H2O Chemicals Masses RFM Moles Ratio H2 H2O 8g ? 1+1 =2 4 moles 4moles 2:2 so 1:1 (1 mole of H2 = 1 mole of H2O) 5) Finally use the mole equation to work out the mass of the H2O produced. Moles = mass/RFM use the triangle to rearrange to get mass = moles x RFM RFM of H2O = H+H+O = 1+1+16 = 18 so Mass = moles x RFM = 4moles x18 = 72g How to Sheet: Calculate masses using equations: Example question: 8g of Hydrogen is reacted with Oxygen how much water will it produce? Method 1) Write out equation and balance e.g. H2 + O2 H2O 2H2 + O2 2H2O 2) Put in the masses given : 2H2 + O2 2H2O Chemicals Masses RFM Moles Ratio 8g H2 8g 1+1 = 2 ? ? H2O ? ? ? ? 3) Use the mass to work out the number of moles eg Moles = Mass /RFM, so Moles of H2 = 8g/2 = 4moles of H2O put this in to the equation grid Chemicals Masses RFM Moles Ratio H2 8g 1+1 = 2 4 moles H2O ? 4) Use equation to work out ratio, then use this to work out the number of moles of H2O. As it is 1 to 1, if we have 4 moles of H2 then we get 4 moles of H2O Chemicals Masses RFM Moles Ratio H2 H2O 8g ? 1+1 =2 4 moles 4moles 2:2 so 1:1 (1 mole of H2 = 1 mole of H2O) 5) Finally use the mole equation to work out the mass of the H2O produced. Moles = mass/RFM use the triangle to rearrange to get mass = moles x RFM RFM of H2O = H+H+O = 1+1+16 = 18 so Mass = moles x RFM = 4moles x18 = 72g





