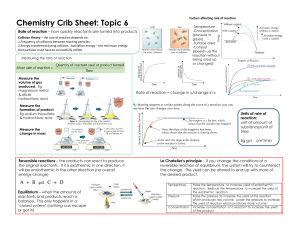
Rate of Reaction Learning Objectives • Describe and evaluate practical methods for investigating the rate of reaction, including change in mass of a reactant or a product and the formation of a gas Types of Reactions • Respiration • Combustion [when you burn anything in the presence of oxygen] • Displacement [halide displacement, when a more reactive halogen displaces a less reactive one] • Photosynthesis • Neutralisation [acid and alkali react] • Precipitation reaction [two aqueous solutions mix to form a precipitate] • Think of an example of a fast chemical reaction and a slow chemical reaction. What is rate of reaction? • Reactions that occur at di erent speeds • Change that occurs per unit time • Time measured in • Seconds • Minutes • Hours • Months ff • Days In what context would speed of a reaction matter? Observing Rate of Reaction Reaction between Zinc and Hydrochloric Acid ff Products? Zinc chloride and hydrogen Observations? E ervescence/bubbling Chemical Equation, with state symbols? Zn (s) + 2 HCl (aq) —> ZnCl2 (aq)+ H2 (g) How would you measure the rate of this reaction? Observing Rate of Reaction • You could measure the rate of reaction, by measuring: • The amount of zinc used per minute • The amount of hydrochloric acid used per minute • The amount of zinc chloride produced • The amount of hydrogen produced Observing Rate of Reaction • For the reaction, what would be the easiest way to observe the rate of reaction using common laboratory apparatus. Zinc Results • How would the volume of hydrogen change over time? • It will increase because the reactants will react and continue produce hydrogen • Will the volume increase by same amount in each interval of time? • No • Reason for answer? • Because the reactants are being used up • What would the graph look like? A: Initially, the reaction will be the fastest Or most amount of hydrogen produced or the graph will be the steepest because initially maximum amount of reactants are available B: the gradient of the graph decreases or graph becomes less steep because reactants are being used up C: either one or both the reactants have been used up Reactant that nishes up rst? Limiting reagent The one the remains behind? In excess fi fi Rate of Reaction = volume of hydrogen / time = 40 / 5 = 8 cm3 / min Rate of reaction at the second minute = 25/2 = 12.5 cm3/min Understanding the graph Observing Rate of Reaction • Calcium Carbonate + Hydrochloric Acid —> • What are the products formed? • Calcium chloride + carbon dioxide + water • Observations? • E ervescence/ bubbling • Chemical Equation? • CaCO3 (S) + 2 HCl (aq) —> CaCl2 (aq) + CO2 (g) + H2O (l) • The mass of reactants = mass of products [some mass of products is being lost] • As the reaction proceeds what will happen to the mass of the container with the reactants in it? ff • It will decrease Marble chips - calcium carbonate Mass of container will start to decreased as carbon dioxide is being produced and lost, as the reaction proceeds Cotton wool - it allows the carbon dioxide to escape but makes sure the reactants don’t With time the mass of the container will start to decrease Suitable for heavier gases but not for lighter ones, e.g. hydrogen gas Results • How would the mass change over time? • Decrease • Will the mass decrease by same amount in each interval of time? • No • Reason for answer? • Reactants are being used up • What would the graph look like? Results