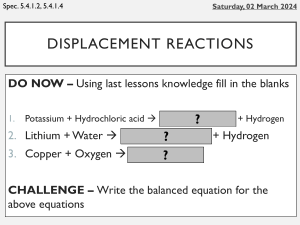
Metal Displacement Reactions Equipment Test tubes and test tube rack Copper metal Magnesium metal Zinc metal Iron nail Copper sulfate solution Magnesium sulfate solution Zinc sulfate solution Iron (II) sulfate solution M M M M Safety Wear a labcoat and safety glasses throughout the experiment and also when you or others are cleaning up at the end of the experiment. If you spill any chemicals on your skin, immediately wash them off with water. If you spill any on the desk, clean it carefully with a paper towel or cloth. Method Add 3 cm of metal sulfate solution into 3 test tubes. Add the other Three metals one at a time and write down any changes to: The Colour of Metal The Colour of Liquid The Temperature The formation of bubbles or fizzing. Repeat steps 1 + 2 again using a different solution a set of three other metals until you have completed all reactions. Turn Over Paper Results Copper Sulfate Magnesium Sulfate Zinc Sulfate Iron Sulfate Copper (Cu) x Magnesium (Mg) x Zinc (Zn) x Iron (Fe) x Conclusion Copper displaces _____________________________________________________ . Magnesium displaces _____________________________________________________ . Zinc displaces _____________________________________________________ . Iron displaces _____________________________________________________ . Write down the Order of Reactivity _____________________ Most Reactive _____________________ _____________________ _____________________ Least Reactive