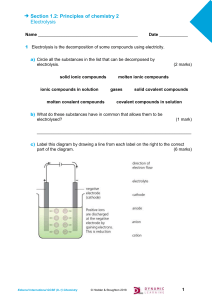
CHEMISTRY – F4- ELECTROLYSIS Lab Session – 2 Name: Gas will not conduct electricity in any form To conduct electricity in solid and liquid you need charged particles. In solid: delocalised mobile electrons In solutions: Free moving ions (cations and anions) liquid Solid conductors Insulators Electrolyte Non-Electrolyte Most covalent compounds do not conduct electricity as they have no freely moving charged particles to carry the current 1|P A G E CLASS WORK msSripali Electrolysis of Molten Compounds Lead(II) bromide is a binary ionic compound- it is a compound consisting of just two elements joined together by ionic bonding When these compounds are heated beyond their melting point, they become molten What happens here? https://www.youtube.com/watch?v=7uIIq_Ofzgw&list=RDCMUCS3wWlfGUijnRIf745lRl2A&index=1&ab_channel=FuseSch ool-GlobalEducationFuseSchool-GlobalEducationVerified 2|P A G E CLASS WORK msSripali KEY TERMS: 3|P A G E CLASS WORK msSripali How to predict the products To predict the products of any binary molten compound Step 1: first identify the ions present Step 2:The positive ions will migrate toward the cathode and the negative ions will migrate towards the anode Step 3: cathode product will always be the metal and the product formed at the anode will always be the non-metal 4|P A G E CLASS WORK msSripali https://www.youtube.com/watch?v=87K8QsMl8nc&ab_channel=FuseSchool-GlobalEducationFuseSchoolGlobalEducationVerified practicals : https://www.youtube.com/watch?v=4x2ZCSr23Z8&ab_channel=FuseSchool-GlobalEducationFuseSchoolGlobalEducationVerified 5|P A G E CLASS WORK msSripali 6|P A G E CLASS WORK msSripali 7|P A G E CLASS WORK msSripali Teams- intendent work- 15 mins 8|P A G E CLASS WORK msSripali Task 1: Set up the given circuit and record your observation Distilled water Theory 9|P A G E CLASS WORK msSripali Task 2: To the same beaker add H2SO4 solution and record your observations Dilute sulfuric acid Task 3 10 | P A G E CLASS WORK msSripali Sodium chloride solution 11 | P A G E CLASS WORK msSripali