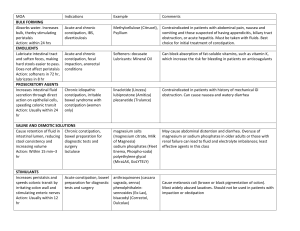
NRG Management of Constipation Version 1.0 Goal: Develop an evidence-based template for the management of constipation. Practical Considerations Remember to review the protocol for any prohibited medications as some medications may be prohibited due to drug interactions Supportive care should follow institutional standards when permitted per the protocol Local complications from constipation may include fecal impaction, overflow diarrhea, gastrointestinal obstruction or perforation, hemorrhoids, rectal prolapse, and anal tears or rectal bleeding Additional complications may include halitosis, early satiety, nausea and vomiting, and gastroesophageal reflux Consider and address possible causes of functional constipation o Positive family history o Low levels of dietary fiber or poor food and fluid intake o Low levels of physical activity o Lack of privacy, need for assistance during toileting Consider causes of secondary constipation and modify therapy as indicated o Opioid-induced o Other medications (e.g., antacids, diuretics, anti-emetics, anticholinergics, iron supplements, vinca alkaloids) o Metabolic problems (e.g., dehydration, hypercalcemia, hypokalemia, hypothyroidism, diabetes) o Neurological or psychiatric diseases (e.g., dementia, depression) o Gastrointestinal diseases (e.g., diverticulosis, irritable bowel syndrome) o Structural issues (e.g., abdominal or pelvic mass, radiation fibrosis, peritoneal carcinomatosis) Opioid-induced constipation o All patients prescribed an opioid should be started on concomitant laxative therapy, unless contraindicated by pre-existing diarrhea o Osmotic or stimulant laxatives are preferred o Bulk laxatives such as psyllium are not recommended o If unresolved with conventional laxatives, peripheral opioid antagonists (methylnaltrexone or naloxegol) may be of value Titrate to a bowel movement every 1-2 days (or the patient’s baseline) Therapeutic Approach to Constipation in Patients with Cancer Preventative Measures Set goals of treatment and explain to patient and family (soft stools, ease of defecation, bowel movement every 1-2 days, adjusted per individual bowel habits) Patients taking daily opioids almost always require agents for management of constipation Prophylactic medications o Stimulant laxative (e.g., senna 2 tablets daily; maximum 8 tablets per day) o Osmotic laxative (e.g., polyethylene glycol 17 grams = 1 heaping tablespoon in 8 oz of water PO 1-2 times daily o May need to increase dose of laxative when increasing dose of opioids Maintain adequate fluid intake While maintaining adequate dietary fiber is recommended, supplemental medicinal fiber (e.g. psyllium) is unlikely to control opioid-induced constipation and may worsen constipation Exercise, if tolerated Page 2 Constipation Treatment Assess for cause and severity of constipation, including impact or other contributing medications Rule out obstruction Titrate laxatives as needed with a goal of one non-forced bowel movement every 1 to 2 days o Stimulant laxative (e.g., senna 2 tablets daily; maximum 8 tablets per day) and/or o Osmotic laxative (e.g., polyethylene glycol 17 grams dissolved in 4-8 oz of beverage PO 1-2 times daily) If senna and polyethylene glycol have not relieved constipation, consider adding another agent o Magnesium hydroxide o Bisacodyl orally or rectally (avoid rectal route in neutropenic or thrombocytopenic patients) o Lactulose o Magnesium citrate If response to laxative therapy has not been sufficient for opioid-induced constipation, consider peripherally acting mu-opioid receptor antagonists (PAMORAs) such as methylnaltrexone, naloxegol, or naldemedine o These agents should not be used in patients with known or suspected mechanical bowel obstruction, recent bowel surgery, transmural bowel metastases, or other processes affecting integrity of GI lumen due to potential increased risk of perforation Bulk laxatives are not preferred in patients with cancer as they require fluid volume and impact wanes over time Increase activity and mobility, within patient limits In general, enemas are only used if oral treatment fails after several days and in order to prevent fecal impaction Contraindications for enema use o Neutropenia or thrombocytopenia o Paralytic ileus or intestinal obstruction o Recent colorectal or gynecological surgery o Recent anal or rectal trauma o Severe colitis, inflammation, or infection of the abdomen o Toxic megacolon o Undiagnosed abdominal pain o Recent radiotherapy to the pelvic area References Davies A, Leach C, Caponero R, et al. MASCC recommendations on the management of constipation in patients with advanced cancer. Support Care Cancer 2020; 28(1): 23-33. Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2018; 29: iv111-iv125. National Comprehensive Cancer Network. Adult Cancer Pain (Version 1.2021). Available from: https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Page 3 Senna Bisacodyl Polyethylene glycol (PEG) Lactulose Magnesium hydroxide Magnesium citrate Methylnaltrexone Naloxegol Drug Specific Considerations Stimulant Laxatives Oral, tablet (8.6 mg sennosides) Best taken in the evening or at bedtime 8.6 mg sennosides/tablet: 2 tablets with the aim of producing a normal daily; maximum 4 tablets twice daily stool next morning Oral, syrup (8.8 mg sennosides/5 mL) 8.8 mg/5 mL syrup: 10 to 15 mL once daily; maximum 15 mL twice daily Oral, tablet (5 mg) 5 to 15 mg once daily Rectal, suppository or enema (10 mg) 10 mg (1 suppository or enema) once daily Osmotic Laxatives Oral, powder or packet (17 g/dose) 17 mg (~1 heaping tablespoon) dissolved in 120 to 240 mL (4-8 oz) of beverage once to twice daily Oral, solution (10 g/15 mL) 10 to 20 grams (15 to 30 mL) daily May increase to 40 grams (60 mL) daily Oral, suspension (milk of magnesia) Magnesium hydroxide 400 mg/5 mL 30 to 60 mL once daily or in divided doses Magnesium hydroxide 800 mg/5 mL 15 to 30 mL once daily or in divided doses Magnesium hydroxide 1,200 mg/5 mL 10 to 20 mL once daily or in divided doses Oral, solution 195 to 300 mL given once or in divided doses Opioid Receptor Antagonists SubQ <38 kg: 0.15 mg/kg 38 to <62 kg: 8 mg 62 to 114 kg: 12 mg >114 kg: 0.15 mg/kg Oral, tablet (12.5 mg, 25 mg) 25 mg once daily, may reduce to 12.5 mg once daily if not tolerated Titrate to effect with a maximum dose of 4 tablets twice daily Suppositories/enemas are contraindicated for patients who are neutropenic or thrombocytopenic Virtually no net gain or loss of sodium and potassium Not absorbed by the small bowel Takes 2-3 days before onset Use cautiously in renal impairment Excessive doses of oral magnesium salts can lead to hypermagnesemia Best taken at bedtime with the aim of producing a normal stool next morning Use cautiously in renal impairment Accumulation of magnesium may lead to magnesium intoxication FDA approved for opioid-induced constipation in adults with advanced illness who are receiving palliative care FDA approved for opioid-induced constipation in adults with chronic noncancer pain Discontinue all maintenance laxative therapy prior to use and reintroduce, as needed after 3 days Significant drug interactions exist Page 4 Naldemedine Oral, tablet (0.2 mg) 0.2 mg once daily FDA approved for opioid-induced constipation in adults with chronic noncancer pain Significant drug interactions exist