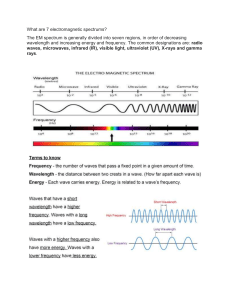
Nature of Light What is light? Light is radiant energy, usually referring to electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. In 1643-1727 Sir Isaac Newton discovered that light coming from the Sun pass through a Glass prism will result into a formation of different colors In the rainbow. And this stream of Particles he calls it COSPUSCLES. At the dawn of 21th century, a Modern well-known scientist, have learned through experimentation that light behaves like a particle at times, and like a wave at other times. The particlelike features are called photons… And this photons travel on a constant speed of 300,000 km/sec (186,000 mi/sec) Theory of Electromagnetic wave theory theorized that this mutual generation and propagation of electric field and magnetic field can be conceived as a form of moving energy carried by what he called as electromagnetic wave. JAMES CLERK MAXWELL Theory of Electromagnetic wave theory a German physicist who applied Maxwell’s theories to the production and reception of radio waves. The unit of frequency of a radio wave - one cycle per second - is named the hertz Heinrich Hertz Theory of Electromagnetic wave theory His contributions to electrical engineering and electrochemistry or due to the fact that he was responsible for introducing the concept of field in physics to describe electromagnetic interaction are enough for him to be highly recognized. Inventor of the Motor, Generator, And transformer Electromagnetic Induction. Michael Faraday Theory of Electromagnetic wave theory made the revolutionary discovery that a wire carrying electric current can attract or repel another wire next to it that’s also carrying electric current. The attraction is magnetic, but no magnets are necessary for the effect to be seen. He went on to formulate Ampere’s Law of Electromagnetism and produced the best definition of electric current during his time. Andre-Marie Ampere Theory of Electromagnetic wave theory a Danish physicist and chemist who discovered that the electric current in a wire can deflect a magnetized compass needle, a phenomenon the importance of which was rapidly recognized and which inspired the development of electromagnetic theory. Hans Christian Oersted Hans C. Oersted’s experiment The Basic Principles of EM Wave Theory After years of rigorous studies and experiments, the following principles came about to explain the Electromagnetic Wave Theory. 1. Many natural phenomena exhibit wave-like behaviors. All of them – water waves, earthquake waves, and sound waves require a medium to propagate. These are examples of mechanical waves. 2. Light can also be described as a wave – a wave of changing electric and magnetic fields that propagate outward from their sources. These waves, however, do not require a medium to propagate. 3. They propagate at 300,000,000 meters per second through a vacuum. 4. Electromagnetic waves are transverse waves. In simpler terms, the changing electric and magnetic fields oscillate perpendicular to each other and to the direction of the propagating waves. These changing electric and magnetic fields generate each other through Faraday’s Law of Induction and Ampere’s Law of Electromagnetism. These changing fields dissociate from the oscillating charge and propagate out into space at the speed of light. 5. When the oscillating charge accelerates, the moving charge’s electric fields change, too. Albert Einstein (1879-1955) Light as a Wave… Parts of a wave: Depending on the frequency, Light waves can be set on a specific spectrum like: 1. 2. 3. 4. Micro-waves X-rays Ultraviolet rays Gamma Waves Interfence It is a phenomena where as two or more wavelengths overlap in space and this is called SUPERPOSITION. There are TWO TYPES OF INTERFERENCE 1. CONSTRUCTIVE INTERFERENCE 2. DESTRUCTIVE INTERFERENCE Diffraction Phenomenon were light waves bend around an obstacle Polarization is a property of waves that can oscillate with more than one orientation. Electromagnetic waves, such as light, and gravitational waves exhibit polarization Light as a RAY Reflection Reflection: is incident on the surface equals the angle at which it is reflected. 2 types of Reflection SPECULAR REFLECTION - light rays bounce off a smooth surface and reflected rays follow a uniform path DIFFUSED REFLECTION - Light rays bounce off a rough surface causing the light in a non-uniformed path. Refraction is a phenomenon that often occurs when waves travel from a medium with a given reflective material to a medium with another at an angle Practice Exercises 1. the energy emitted by visible light is considered as bundles of electromagnetic energy called photons. If a 100W light bulb bas an ave. wavelength of 5.3X10^-7m. How many photons per second does the light emit? 2) Microwaves are also used to cook food. If a microwave oven radiates microwaves with a wavelength of 2.2cm how much energy does it emit? Note: science book (grd10 pg75) Answers: the energy emitted by visible light is considered as bundles of electromagnetic energy called photons. If a 100W light bulb bas an ave. wavelength of 5.3X10^-7m. How many photons per second does the light emit? Given: Wavelength (𝜆): 3.3𝑥10−7𝑚 Speed of EM: 3𝑥108 𝑚/𝑠 Frequency: __________Hz 𝑓 = 𝑐/𝜆 𝑓 = 3𝑥108 𝑚/𝑠 ÷ 5.3𝑥10−7 𝑚 𝑡ℎ𝑒𝑟𝑒𝑓𝑜𝑟𝑒: 𝑡ℎ𝑒 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑝ℎ𝑜𝑡𝑜𝑛𝑠 𝑝𝑒𝑟 𝑠𝑒𝑐𝑜𝑛𝑑 𝑜𝑟 ℎ𝑧 𝑡ℎ𝑒 𝑙𝑖𝑔ℎ𝑡 𝑏𝑢𝑙𝑏 𝑒𝑚𝑖𝑡 𝑖𝑠 𝑎𝑏𝑜𝑢𝑡 [5.66𝑥1014 𝐻𝑧.] Answers: Microwaves are also used to cook food. If a microwave oven radiates microwaves with a wavelength of 2.2cm how much energy does it emit? Given: Wavelength (𝜆): 2.2𝑐𝑚 → 2.2𝑥10−2 𝑚 Speed of EM: 3𝑥108 𝑚/𝑠 Frequency: __________Hz Energy : _____________ Joules 𝑓 = 𝑐/𝜆 𝑓 = 3𝑥108 𝑚/𝑠 ÷ 2.2𝑥10−2 𝑚 𝑓 = 1.36𝑥1010 𝐻𝑧 𝐸=ℎ 𝑓 𝐸 = 6.63𝑥10−34 1.36𝑋1010 𝐻𝑧 𝑇ℎ𝑒𝑟𝑒𝑓𝑜𝑟𝑒 𝑡ℎ𝑒 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑒𝑛𝑒𝑟𝑔𝑦 𝑡ℎ𝑒 𝑚𝑖𝑐𝑟𝑜𝑤𝑎𝑣𝑒 𝑒𝑚𝑖𝑡𝑠 𝑖𝑠 𝑎𝑏𝑜𝑢𝑡 [9.01𝑥10−24 𝐽𝑜𝑢𝑙𝑒𝑠] or 5.63125𝑥10−5 eV The different parts of the spectrum of light. Radiowaves (am) Radio waves were first predicted by mathematical work done in 1867 by Scottish mathematical physicist James Clerk Maxwell Radiowaves have a every low energy but has a higher wavelength. Radio waves in vacuum travel at the speed of light. When passing through a material medium, they are slowed according to that object's permeability and perm ittivity. Air is thin enough that in the Earth's atmosphere radio waves travel very close to the speed of light. Radio Broadcasting: types of Modulation. AM (amplitude Modulation) Developed by Lee de Forest it was the first method developed for making audio radio transmissions but possesses a medium wavelength. Due to this AM transmissions are much more susceptible to interference than FM and digital sounds thus AM Broadcasting tend to specialize in spoken-word formats, such as talk radio, all news and sports. FM ( Frequency modulation) Invented by Edwin Armstrong (1933) is a broadcasting capable of better sound than AM broadcasting Microwave Microwaves travel solely by line-of-sight paths; unlike lower frequency radio waves, they do not travel as ground waves which follow the contour of the Earth, or reflect off the ionosphere (skywaves).Although at the low end of the band they can pass through building walls enough for useful reception, usually rights of way cleared to the first Fresnel zone are required. Therefore, on the surface of the Earth, microwave communication links are limited by the visual horizon to about 30–40 miles (48–64 km). Microwave uses: Microwave technology is extensively used for point-topoint telecommunications (i.e. non-broadcast uses). Microwaves are especially suitable for this use since they are more easily focused into narrower beams than radio waves Microwaves are used in spacecraft communication, and much of the world's data, TV, and telephone communications are transmitted long distances by microwaves between ground stations and communications satellites. Microwaves are also employed in microwave ovens and in radar technology. Infrared: • Infrared radiation is electromagnetic radiation (EMR) with longer wavelengths than those of visible light, and is therefore invisible to the human eye. • nfrared radiation is popularly known as "heat radiation", but light and electromagnetic waves of any frequency will heat surfaces that absorb them. Infrared light from the Sun accounts for 49% of the heating of Earth, with the rest being caused by visible light that is absorbed then re-radiated at longer wavelengths. • Frederick William Herschel – helped to discover infrared. Visible Spectrum The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. Colors that can be produced by visible light of a narrow band of wavelengths (monochromatic light) are called pure spectral colors. The various color ranges indicated in the illustration are an approximation: The spectrum is continuous, with no clear boundaries between one color and the next. spectrum into seven named colors: red, orange, yellow, green, blue, indigo, and violet. Spectroscopy is the study of objects based on the spectrum of color they emit, absorb or reflect. Spectroscopy is an important investigative tool in astronomy, where scientists use it to analyze the properties of distant objects. Typically, astronomical spectroscopyuses high-dispersion diffraction gratings to observe spectra at very high spectral resolutions. Helium was first detected by analysis of the spectrum of the sun. Chemical elements can be detected in astronomical objects by emission lines and absorption lines. Ultraviolet (UV) "Ultraviolet" means "beyond violet" (from Latin ultra, "beyond"), violet being the color of the highest frequencies of visible light. Ultraviolet has a higher frequency than violet light. UV radiation was discovered in 1801 when the German physicist Johann Wilhelm Ritter is an electromagnetic radiation with a wavelength from 10 nm to 400 nm, shorter than that of visible lightbut longer than X-rays. UV radiation is present in sunlight constituting about 10% of the total light output of the Sun. Practical uses of UV: mercury-vapor lamps, Welding tools (electrical arch) tanning lamps, and black lights X-ray X-ray wavelengths are shorter than those of UV rays and typically longer than those of gamma rays X-radiation is referred to with terms meaning Röntgen radiation, after the German scientist Wilhelm Röntgen X-ray photons carry enough energy to ionize atoms and disrupt molecular bonds. This makes it a type of ionizing radiation, and therefore harmful to living tissue. A very high radiation dose over a short period of time causes radiation sickness, while lower doses can give an increased risk of radiation-induced cancer. In medical imaging this increased cancer risk is generally greatly outweighed by the benefits of the examination. The ionizing capability of X-rays can be utilized in cancer treatment to kill malignant cells using radiation therapy X-ray uses: Gamma Rays (y) Gamma rays (gamma radiation) denoted by the lower case greek letter Gamma (y) are penetrating electromagnetic radiation. Paul Villard a French chemist and physicists discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903 Ernest Rutherford named this radiation “gamma Rays”. Natural source of Gamma Radiation: Quasar (footnote: defination) Formula on getting the speed of light on a Refracting Medium N= C / V V=C/V GIVEN: N= Refracting Medium C = 3.00 x10^8 m/s (speed of Light in a Vacuum) V = speed of light in the medium Sample Problem: 1. What is the speed of light in a 30%sugar solution? Given: Nsugar at 30% = 1.38 Speed of light , C =3.00x 10^8m/s MORE PROBLEMS TO SOLVE: 1. What is the speed of Light in an 80% sugar solution? 2. What is the Speed of Light in an Diamond? 3. What is the Speed of Light in an NaCl Solution? 4. What is the Speed of Light in a Glass of water at 20’C ?